4 | | Board Risk Oversight Our Board of Directors believes that a fundamental part of risk management is understanding the risks that we face, monitoring these risks and adopting appropriate control and mitigation of these risks. As stated in our Corporate Governance Principles, our Board of Directors and its committees are responsible for “reviewing the Company’s risk framework and governance and management’s exercise of its responsibility to assess, monitor and manage the Company’s significant risk exposures.” Our Board of Directors oversees the management of material risks facing the Company. Biogen is committed to fostering a company culture of risk-adjusted decision-making without constraining reasonable risk-taking and innovation. Our Board of Directors and its committees oversee our efforts to foster this culture. Our Board of Directors regularly receives information about our material strategic, operational, financial and compliance risks and management’s response to, and mitigation of, such risks. In addition, our risk management systems, including our risk assessment processes, internal control over financial reporting, compliance programs and internal and external auditing procedures, are designed to inform management and our Board of Directors about our material risks. As part of its risk oversight function, our Board of Directors and its committees review this framework, its operation and our strategies for generating long-term value for our stockholders to ensure that such strategies will not motivate management to take excessive risks. Our Board of Directors also reviews enterprise risks and discusses them with our management, including issues relevant to our business, reputation and strategy, including intellectual property risk, pipeline and business development, pricing and patient access, legal and regulatory matters and manufacturing. In addition, our Board of Directors and its committees oversee elements of our culture. Management updates our C&MD Committee on our compensation practices and progress against strategies and objectives in the areas of management and leadership development and diversity as well as steps taken to address matters such as inappropriate workplace behavior, including harassment and retaliation. In addition, our Audit Committee is responsible for the oversight of our compliance program. Audit Committee Matters | | | | | | | | | 23 | | 
| |  |
| | | | 3 | | Board of Directors (continued) |
In determining the allocation of risk oversight responsibilities, our Board of Directors and its committees generally oversee material risks within their identified areas of concern. Our Board of Directors and each of its committees meet regularly with management to ensure that management is exercising its responsibility to identify relevant risks and is adequately assessing, monitoring and taking appropriate action to mitigate risk. In the event a committee receives a report from members of management on areas of material risk to the Company, the Chair of the relevant committee reports on the discussion to the full Board of Directors at the next Board of Directors meeting. This enables our Board of Directors and its committees to coordinate their oversight of risk and identify risk interrelationships. Our independent Chairman of the Board promotes effective communication and consideration of matters presenting significant risks to the Company through his role in developing our Board of Directors’ meeting agendas, advising committee chairs, chairing meetings of the independent directors and facilitating communications between independent directors and our Chief Executive Officer. A summary of the key areas of risk oversight responsibility of our Board of Directors and each of its committees is set forth below: | | | | | | | Board or Committee | | Area of Risk Oversight | Board | | • Corporate and commercial strategy and execution, pricing and reimbursement, competition, reputational, environmental, health and sustainability and other material risks • Research and development activities, clinical development, drug safety and intellectual property • Material government and other investigations and litigation • Risk governance framework and infrastructure designed to identify, assess, manage and monitor the Company’s material risks • Risk management policies, guidelines and practices implemented by Company management | | | Audit | | • Financial, accounting, disclosure, corporate compliance, distributors, insurance, capital, credit, anti-bribery and anti-corruption matters and other risks reviewed in its oversight of the internal audit and corporate compliance functions • Information technology and cybersecurity risks | | | Compensation and Management Development | | • Workforce matters, including harassment • Compensation policies and practices, including whether such policies and practices balance risk-taking and rewards in an appropriate manner as discussed further below | | | Corporate Governance | | • Corporate governance and board succession, director independence, potential conflicts of interest and related party transactions involving directors and executive officers | | |
Compensation Risk Assessment The Compensation Discussion and Analysis (CD&A) section of this Proxy Statement describes our compensation policies, programs and practices for our named executive officers. Our goal-setting, performance assessment and compensation decision-making processes described in the CD&A generally apply to all employees. We offer a limited number of short-term cash incentive plans, with employees eligible for either our annual bonus plan or a sales incentive compensation plan. Except in limited circumstances, no employee is eligible to participate in more than one cash incentive plan at any time. Our annual bonus plan is consistently maintained for all participants globally, with the same Company performance goals, payout levels (as a percentage of target) and administrative provisions regardless of the participant’s job level, location or function in the Company. We also have a long-term incentive program that provides different forms of awards depending upon an employee’s level but is otherwise consistent throughout the Company. In the CD&A, we describe the risk-mitigation controls for our executive compensation programs. These controls include C&MD Committee review and approval of the design, goals and payouts under our annual bonus plan and long-term incentive program and each executive officer’s compensation (or, in the case of our Chief Executive Officer’s compensation, a recommendation of that compensation to our Board of Directors for its approval). In addition, we review the processes, controls and design of our sales incentive compensation plans. | | | | | | | | | 24 | | 
| |  |
| | | | 3 | | Board of Directors (continued) |
The C&MD Committee, working with its independent compensation consultant, also conducts an annual assessment of potential risks related to our compensation policies, programs and practices. Among other factors, this risk assessment considers the form of compensation (i.e., award type, fixed versus variable and short-term versus long-term), pay alignment, performance measures and goals, payout maximums, vesting periods and C&MD Committee oversight and independence. This assessment is focused on (1) having an appropriate balance in our program structure to mitigate compensation-related risk with cash versus stock, short-term versus long-term measurement and financial versus non-financial goals; and (2) policies and practices to mitigate compensation-related risk including recoupment of compensation, stock ownership guidelines, equity administration rules and insider-trading and hedging prohibitions. Based on our assessment, we believe that, through a combination of risk-mitigating features and incentives guided by relevant market practices and Company-wide goals, our compensation policies, programs and practices do not create risks that are reasonably likely to have a material adverse effect on the Company. | | | | | | | | | 25 | | 
| |  |
STOCK OWNERSHIP The following table and accompanying notes provide information about the beneficial ownership of our common stock by: each stockholder known by us to be the beneficial owner of more than 5% of our common stock; each of our named executive officers; each of our directors and nominees for director; and all of our directors and executive officers as a group. Except as otherwise noted, the persons identified have sole voting and investment power with respect to the shares of our common stock beneficially owned. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to the shares. Except as otherwise noted, the information below is as of April 5, 2021 (Ownership Date). Unless otherwise indicated in the footnotes, the address of each of the individuals named below is: c/o Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. | | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Shares
Owned(1) | | Shares Subject to Options and Stock Units(2) | | Total Number of Shares Beneficially Owned(1) | | Percentage of Outstanding Shares(3) | 5% Stockholders | | | | | | | | | | | | | | | | | | | | | PRIMECAP Management Company(4) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | | 15,822,066 | | | | | — | | | | | 15,822,066 | | | | | 10.28 | % | BlackRock, Inc.(5) 55 East 52nd Street New York, NY 10055 | | | | 13,419,601 | | | | | — | | | | | 13,419,601 | | | | | 8.7 | % | The Vanguard Group(6) 100 Vanguard Boulevard Malvern, PA 19355 | | | | 11,896,510 | | | | | — | | | | | 11,896,510 | | | | | 7.73 | % | Named Executive Officers | | | | | | | | | | | | | | | | | | | | | Michel Vounatsos | | | | 53,072 | | | | | — | | | | | 53,072 | | | | | * | | Michael R. McDonnell | | | | — | | | | | — | | | | | — | | | | | — | | Alfred W. Sandrock, Jr. | | | | 17,841 | | | | | — | | | | | 17,841 | | | | | * | | Susan H. Alexander | | | | 41,576 | | | | | — | | | | | 41,576 | | | | | * | | Chirfi Guindo | | | | 6,006 | | | | | — | | | | | 6,006 | | | | | * | | Jeffrey D. Capello(7) | | | | 3,118 | | | | | — | | | | | 3,118 | | | | | * | | Directors | | | | | | | | | | | | | | | | | | | | | Alexander J. Denner(8) | | | | 655,064 | | | | | 890 | | | | | 655,954 | | | | | * | | Caroline D. Dorsa | | | | 20,207 | | | | | 890 | | | | | 21,097 | | | | | * | | Maria C. Freire | | | | — | | | | | — | | | | | — | | | | | — | | William A. Hawkins | | | | 1,115 | | | | | 890 | | | | | 2,045 | | | | | * | | William D. Jones | | | | — | | | | | — | | | | | — | | | | | — | | Nancy L. Leaming | | | | 12,098 | | | | | 890 | | | | | 12,988 | | | | | * | | Jesus B. Mantas | | | | 2,053 | | | | | 890 | | | | | 2,943 | | | | | * | | Richard C. Mulligan | | | | 12,064 | | | | | 890 | | | | | 12,954 | | | | | * | | Robert W. Pangia(9) | | | | 19,742 | | | | | 890 | | | | | 20,632 | | | | | * | | Stelios Papadopoulos(10) | | | | 33,301 | | | | | 1,470 | | | | | 34,771 | | | | | * | | Brian S. Posner | | | | 6,870 | | | | | 890 | | | | | 7,760 | | | | | * | | Eric K. Rowinsky | | | | 16,179 | | | | | 890 | | | | | 17,069 | | | | | * | | Stephen A. Sherwin | | | | 15,438 | | | | | 890 | | | | | 16,328 | | | | | * | | Executive officers and directors as a group (21 persons)(11) | | | | 926,695 | | | | | 10,370 | | | | | 937,065 | | | | | * | |
| * | Represents beneficial ownership of less than 1% of our outstanding shares of common stock. |
| | | | | | | | | 26 | | 
| |  |
| | | | 4 | | Stock Ownership (continued) |
Audit and Other Fees
| (1) | The following table shows fees for professional audit services billed to us by PwC for the auditshares described as “owned” are shares of our annual consolidated financial statementscommon stock directly or indirectly owned by each listed person, rounded up to the nearest whole share. |
| (2) | Includes RSUs that will vest within 60 days of the Ownership Date. |
| (3) | The calculation of percentages is based upon 150,554,556 shares outstanding on the Ownership Date, plus for each of the years endedindividuals listed above the shares subject to RSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and Stock Units.” |
| (4) | Based solely on information as of December 31, 2018,2020, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 12, 2021, which also indicates that it has sole voting power over 15,443,182 shares and sole dispositive power over 15,822,066 shares. |
| (5) | Based solely on information as of December 31, 2017, and fees billed to us2020, contained in a Schedule 13G/A filed with the SEC by PwC for other services provided during 2018 and 2017: | | | | | | | | | | | | Fees (amounts in thousands) | | 2018 | | | 2017 | | Audit fees | | $ | 5,177.6 | | | $ | 5,036.3 | | Audit-related fees | | | 302.0 | | | | 281.2 | | Tax fees* | | | 609.0 | | | | 381.0 | | All other fees | | | 322.1 | | | | 7.1 | | Total | | $ | 6,410.7 | | | $ | 5,705.6 | |
* | Includes tax compliance fees of approximately $0.1 million in 2018 and 2017.
|
Audit feesare fees for the audit of our 2018 and 2017 consolidated financial statements included in our Annual ReportsBlackRock, Inc. on Form10-K, reviews of our condensed consolidated financial statements included in our Quarterly Reports on Form10-Q, review of the consolidated financial
statements incorporated by reference into our outstanding registration statements and statutory audit fees in overseas jurisdictions.
Audit-related fees are feesJanuary 29, 2021, which also indicates that principally relate to assurance and related services that are also performed by our independent registered public accounting firm. More specifically, these services include audits of employee benefit plan information, accounting consultations, due diligence and audits in connection with business development activity, internal control reviews and attest services related to financial reporting that are not required by statute or regulation.
Tax feesare fees for tax compliance and planning services. The increase in fees incurred in 2018 is driven by incremental support for international tax matters.
All other feesin 2018 include $0.3 million related to consultation servicesit has sole voting power with respect to supply chain optimization strategies for11,672,922 shares and sole dispositive power with respect to 13,419,601 shares.
|
| (6) | Based solely on information as of December 31, 2020, contained in a Schedule 13G/A filed with the development of new productsSEC by The Vanguard Group on February 10, 2021, which also indicates that it has sole dispositive power with respect to 11,213,323 shares, shared voting power with respect to 259,934 shares and services. All other fees in 2018shared dispositive power with respect to 638,187 shares. |
| (7) | Mr. Capello ceased to be our Executive Vice President and 2017 also includelicense fees for aweb-based accounting research tool. Policy onPre-Approval of Audit andNon-Audit Services
Our Audit Committee has the sole authority to approve the scope of the audit and any audit-related services as well as all audit fees and terms. Our Audit Committee mustpre-approve any audit andnon-audit services provided by our independent registered public accounting firm. Our Audit Committee will not approve the engagement of the independent registered public accounting firm to perform any services that the independent registered public accounting firm would be prohibited from providing under applicable securities laws, Nasdaq requirements or Public Company Accounting Oversight Board rules. In assessing whether to approve the use of our independent registered public accounting firm to provide permittednon-audit services, our Audit Committee tries to minimize relationships that could appear to impair the objectivity of our independent registered public accounting firm. Our Audit Committee will approve permittednon-audit services by our independent registered public accounting firm only when it will be more effective or economical to have such services provided by our independent registered public accounting firm than by another firm.
Our Audit Committee annually reviews andpre-approves the audit, audit-related, tax and other permissiblenon-audit services that can be provided by the independent registered public accounting firm. After the annual review, any proposed services exceedingpre-set levels or amounts, or additional services not previously approved requires separatepre-approval by our Audit Committee or the Chair of our Audit Committee. Anypre-approval decision made by the Chair of our Audit Committee is reported to our Audit Committee at the next regularly scheduled Audit Committee meeting. Our Chief Financial Officer on August 15, 2020, and ourseparated from the Company on September 15, 2020.
|
| (8) | Includes 643,000 shares beneficially owned by funds and accounts managed by Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital). Dr. Denner is the Chief AccountingInvestment Officer can approve up to an additional $50,000 inof Sarissa Capital and ultimately controls the aggregate per calendar year for categories of services that our Audit Committee (or the Chair through its delegated authority) haspre-approved. Allfunds and accounts managed by Sarissa Capital. By virtue of the services provided by PwC during 2018 and 2017 wereforegoing, Dr. Denner may be deemed to indirectly beneficially own (as that term is defined in Rule pre-approved13d-3 in accordance with this policy.
| | | | | 29 | |  | |  |
| | | 5 | | Executive Compensation Mattersof the Exchange Act) the 643,000 shares that those entities beneficially own. Dr. Denner disclaims beneficial ownership of these shares except to the extent of any pecuniary interest therein.
|
| (9) | | | | | | | | | | | | | Proposal 3 – Advisory Vote on Executive Compensation
| | | | | | | |
Our Compensation Discussion and Analysis,Includes 16,000 shares beneficially owned by Robin Drive, LLC, of which appears below, describes our executive compensation programs andMr. Pangia’s wife is the compensation decisions that our C&MD Committee andTrustee. Mr. Pangia is retiring from our Board of Directors, made with respect to the 2018 compensation of our named executive officers. As required pursuant to Section 14Aeffective as of the Exchange Act, our BoardAnnual Meeting.
|
| (10) | Includes 28,206 shares held in limited liability companies of Directorswhich Dr. Papadopoulos is asking that stockholders cast anon-binding, advisory vote FOR the following resolution:sole manager. |
| (11) | “RESOLVED, that the compensation paid to the Company’s named executive officers, as disclosed pursuant to Item 402 of RegulationS-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.”Includes 688,175 shares held indirectly through trusts, funds, defined benefit plans or limited liability companies.
Our Board of Directors is asking that our stockholders support this proposal. Although the vote you are being asked to cast isnon-binding, we value the views of our stockholders, and our C&MD Committee and our Board of Directors will consider the outcome of the vote when making future compensation decisions for our named executive officers.
|
| | | | | | | | | As we describe in our Compensation Discussion and Analysis, our executive compensation programs embody apay-for-performance philosophy that supports our business strategy and aligns the interests of our executives with those of our stockholders. In particular, our compensation programs reward financial, strategic and operational performance and the goals set under our plans support our short- and long-range plans. In addition, to discourage excessive risk taking, we maintain policies for stock ownership and our equity and annual bonus incentive plans have provisions providing for the recoupment of compensation. We also cap payments under our annual bonus plan and we generally require multi-year vesting periods for long-term incentive awards.27
| | We will hold anon-binding, advisory vote of our stockholders on the compensation of our named executive officers every year until the next required stockholder vote on the frequency of such advisory vote. The next stockholder vote on the frequency of such advisory vote is expected to be held at the 2023 annual meeting of stockholders.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE
FOR THE APPROVAL OF THE RESOLUTION SET FORTH ABOVE.
| | | | | 30 | |  | |  |
| | | | | | | Proposal 2 – Ratification of the Selection of Our Independent Registered Public Accounting Firm | | | | | | | |
Our Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the independent registered public accounting firm retained to audit our consolidated financial statements. Our Audit Committee has selected PwC as our independent registered public accounting firm for the fiscal year ending December 31, 2021. PwC has served as our independent registered public accounting firm since 2003. In order to assure continuing auditor independence, our Audit Committee periodically considers whether there should be a rotation of the independent registered public accounting firm. Further, in conjunction with the rotation of the auditing firm’s lead engagement partner required by applicable SEC rules, our Audit Committee and its Chair has in the past been, and in the future will be, directly involved in the selection of PwC’s new lead engagement partner. Our Audit Committee believes at this time that the continued retention of PwC to serve as our independent registered public accounting firm is in the best interest of Biogen and its stockholders. Although stockholder approval of our Audit Committee’s selection of PwC is not required, our Board of Directors believes that it is a matter of good corporate practice to solicit stockholder ratification of this selection. If our stockholders do not ratify the selection of PwC as our independent registered public accounting firm, our Audit Committee will reconsider its selection. Even if the selection is ratified, our Audit Committee always has the ability to change the engagement of PwC if it considers that a change is in Biogen’s best interest. Representatives of PwC will participate in the Annual Meeting, have the opportunity to make a statement if they so desire and be available to respond to appropriate questions. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE RATIFICATION OF THE SELECTION OF PRICEWATERHOUSECOOPERS LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR THE FISCAL YEAR ENDING DECEMBER 31, 2021. | | | | | | | | | 28 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit Committee Report The Audit Committee’s role is to act on behalf of our Board of Directors in the oversight of Biogen’s financial reporting, internal control and audit functions. The roles and responsibilities of the Audit Committee are set forth in the written charter adopted by our Board of Directors, which is posted on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. Management has primary responsibility for the financial statements and the reporting process, including the systems of internal control. In fulfilling its oversight responsibilities, the Audit Committee, among other things: reviewed and discussed with management the audited consolidated financial statements contained in Biogen’s 2020 Annual Report on Form 10-K; discussed with PwC, Biogen’s independent registered public accounting firm, the overall scope and plans for the audit; met with PwC, with and without management present, to discuss the results of its examination, management’s response to any significant findings, its observations of Biogen’s internal control, the overall quality of Biogen’s financial reporting, the selection, application and disclosure of critical accounting policies, new accounting developments and accounting-related disclosures, the key accounting judgments and assumptions made in preparing the financial statements and whether the financial statements would have materially changed had different judgments and assumptions been made and other pertinent items related to Biogen’s accounting, internal control and financial reporting; discussed with representatives of Biogen’s corporate internal audit staff, with and without management present, their purpose, authority, audit plan and reports; reviewed and discussed with PwC the matters required by the Public Company Accounting Oversight Board and the SEC; discussed with PwC its independence from management and Biogen, including the written disclosures and letter concerning independence received from PwC under applicable requirements of the Public Company Accounting Oversight Board. The Audit Committee has determined that the provision of non-audit services to Biogen by PwC is compatible with its independence; provided oversight and advice to management in connection with Biogen’s system of internal control over financial reporting in response to the requirements set forth in Section 404 of the Sarbanes-Oxley Act of 2002 and related regulations. In connection with this oversight, the Audit Committee reviewed a report by management on the effectiveness of Biogen’s internal control over financial reporting; and reviewed PwC’s Report of Independent Registered Public Accounting Firm included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, related to its audit of the effectiveness of internal control over financial reporting. In reliance on these reviews and discussions, the Audit Committee recommended to our Board of Directors that the audited consolidated financial statements be included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, for filing with the SEC. The Audit Committee of our Board of Directors: Caroline D. Dorsa (Chair) William A. Hawkins Nancy L. Leaming Stephen A. Sherwin, M.D. | | | | | | | | | 29 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit and Other Fees The following table shows fees for professional audit services billed to us by PwC for the audit of our annual consolidated financial statements for the years ended December 31, 2020 and December 31, 2019, and fees billed to us by PwC for other services provided during 2020 and 2019: | | | | | | | | | | | | Fees (amounts in thousands) | | 2020 | | | 2019 | | Audit fees | | $ | 5,882.0 | | | $ | 6,080.3 | | Audit-related fees | | | 48.2 | | | | 55.0 | | Tax fees* | | | 392.5 | | | | 641.8 | | All other fees | | | 9.9 | | | | 7.0 | | Total | | $ | 6,332.6 | | | $ | 6,784.1 | |
| * | Includes tax compliance fees of approximately $0.1 million in 2020 and 2019. |
Audit fees are fees for the audit of our 2020 and 2019 consolidated financial statements included in our Annual Reports on Form 10-K, reviews of our condensed consolidated financial statements included in our Quarterly Reports on Form 10-Q, review of the consolidated financial statements incorporated by reference into our outstanding registration statements and statutory audit fees in overseas jurisdictions. Audit-related fees are fees that principally relate to assurance and related services that are also performed by our independent registered public accounting firm. More specifically, these services include audits of employee benefit plan information, accounting consultations, due diligence and audits in connection with business development activity, internal control reviews and attest services related to financial reporting that are not required by statute or regulation. Tax fees are fees for tax compliance and planning services. All other fees in 2020 and 2019 also includelicense fees for a web-based accounting research tool. Policy on Pre-Approval of Audit and Non-Audit Services Our Audit Committee has the sole authority to approve the scope of the audit and any audit-related services as well as all audit fees and terms. Our Audit Committee must pre-approve any audit and non-audit services provided by our independent registered public accounting firm. Our Audit Committee will not approve the engagement of the independent registered public accounting firm to perform any services that the independent registered public accounting firm would be prohibited from providing under applicable securities laws, Nasdaq requirements or Public Company Accounting Oversight Board rules. In assessing whether to approve the use of our independent registered public accounting firm to provide permitted non-audit services, our Audit Committee tries to minimize relationships that could appear to impair the objectivity of our independent registered public accounting firm. Our Audit Committee will approve permitted non-audit services by our independent registered public accounting firm only when it will be more effective or economical to have such services provided by our independent registered public accounting firm than by another firm. Our Audit Committee annually reviews and pre-approves the audit, audit-related, tax and other permissible non-audit services that can be provided by the independent registered public accounting firm. After the annual review, any proposed services exceeding pre-set levels or amounts, or additional services not previously approved requires separate pre-approval by our Audit Committee or the Chair of our Audit Committee. Any pre-approval decision made by the Chair of our Audit Committee is reported to our Audit Committee at the next regularly scheduled Audit Committee meeting. Our Chief Financial Officer and our Chief Accounting Officer can approve up to an additional $50,000 in the aggregate per calendar year for categories of services that our Audit Committee (or the Chair through its delegated authority) has pre-approved. All of the services provided by PwC during 2020 and 2019 were pre-approved in accordance with this policy. | | | | | | | | | 30 | | 
| |  |
| | | | 6 | | Executive Compensation Matters |
| | | | | | | | | | | | Proposal 3 – Advisory Vote on Executive Compensation | | | | | | | |
Our Compensation Discussion and Analysis, which appears below, describes our executive compensation programs and the compensation decisions that our C&MD Committee and our Board of Directors made with respect to the 2020 compensation of our named executive officers. As required pursuant to Section 14A of the Exchange Act, our Board of Directors is asking that stockholders cast a non-binding, advisory vote FOR the following resolution: “RESOLVED, that the compensation paid to the Company’s named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.” Our Board of Directors is asking that our stockholders support this proposal. Although the vote you are being asked to cast is non-binding, we value the views of our stockholders, and our C&MD Committee and our Board of Directors will consider the outcome of the vote when making future compensation decisions for our named executive officers. As we describe in our Compensation Discussion and Analysis, our executive compensation programs embody a pay-for-performance philosophy that supports our business strategy and aligns the interests of our executives with those of our stockholders. In particular, our executive compensation programs reward financial, strategic and operational performance, and the goals set under our plans support our short- and long-range plans. In addition, to discourage excessive risk taking, we maintain policies for stock ownership, and our equity and annual bonus incentive plans have provisions providing for the recoupment of compensation. We also cap payments under our annual bonus plan, and we generally require multi-year vesting periods for long-term incentive awards. We will hold a non-binding, advisory vote of our stockholders on the compensation of our named executive officers every year until the next required stockholder vote on the frequency of such advisory vote. The next stockholder vote on the frequency of such advisory vote is expected to be held at the 2023 annual meeting of stockholders. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE APPROVAL OF THE RESOLUTION SET FORTH ABOVE. | | | | | | | | | 31 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| COMPENSATION DISCUSSION AND ANALYSIS |
This Compensation Discussion and Analysis (CD&A) describes our compensation strategy, philosophy, policies and practices underlying our executive compensation programs for 2020. It also provides information regarding the compensation that was earned by and awarded to our 2020 named executive officers, whom we refer to collectively as “named executive officers” or “NEOs.” Our named executive officers include our current executive officers listed below as well as Jeffrey D. Capello*, our former Executive Vice President and Chief Financial Officer. | | | | | | | | | | | | |  | | | | Michel Vounatsos Chief Executive Officer | | | |  | | | | Susan H. Alexander Executive Vice President, Chief Legal Officer and Secretary | | | | | | | | | | | | | |  | | | | Michael R. McDonnell* Executive Vice President and Chief Financial Officer | | | |  | | | | Chirfi Guindo Executive Vice President, Global Product Strategy and Commercialization | | | | | | | | | | | | | |  | | | | Alfred W. Sandrock, Jr., M.D., Ph.D.** Executive Vice President, Research and Development | | | | | | | | |
| * | Mr. McDonnell was appointed as Executive Vice President and Chief Financial Officer effective August 15, 2020. Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer on August 15, 2020, and separated from the Company on September 15, 2020. |
| ** | Dr. Sandrock was appointed as Executive Vice President, Research and Development on October 1, 2019. Prior to this appointment, Dr. Sandrock served as our Executive Vice President, Chief Medical Officer, and continued in this role, in addition to his duties as Executive Vice President, Research and Development, until January 27, 2020. |
We had a productive and successful 2020 as we continued to execute well against our corporate strategy. Our full year revenue for 2020 was $13.4 billion, a 6% decrease from the prior year primarily due to the entry of multiple TECFIDERA generic entrants in the U.S. with deeply discounted prices compared to TECFIDERA. The generic competition for TECFIDERA significantly reduced our TECFIDERA revenues during the year ended December 31, 2020, and is expected to have a substantial negative impact on our TECFIDERA revenues for as long as there is generic competition. Excluding TECFIDERA in the U.S., our global MS revenue, including OCREVUS royalties, remained relatively stable for 2020, as compared to 2019, demonstrating the resilience of our MS business in a competitive market. We continued our launch of VUMERITY in the U.S., which was the number two MS product and the number one oral in terms of new prescriptions in the U.S. as of December 31, 2020. We maintained our leadership in our SMA business despite increased competition and delays in SPINRAZA doses due, directly or indirectly, to the COVID-19 pandemic. Although our full year 2020 SPINRAZA revenue decreased 2% as compared to 2019, we continued to see growth outside of the U.S., with full year 2020 revenue outside the U.S. growing 9% as compared to 2019, and we believe that SPINRAZA will remain a foundation of care in the treatment of SMA. | | | | | | | | | 32 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| COMPENSATION DISCUSSION AND ANALYSIS
|
This Compensation Discussion and Analysis (CD&A) describes our compensation strategy, philosophy, policies and practices underlying our executive compensation programs for 2018. It also provides information regarding the manner and context in which compensation was earned by and awarded to our 2018 named executive officers listed below, whom we refer to collectively as “named executive officers” or “NEOs.”
Our full year 2020 biosimilars revenue increased 8% as compared to 2019. Although our biosimilars business was negatively impacted by pricing pressure and a slowdown in new treatments and reduced clinic capacity due to the COVID-19 pandemic, we were the leading anti-TNF biosimilar provider in Europe in 2020, and BENEPALI was the #1 prescribed etanercept product across Europe. We also made significant progress toward building a multi-franchise portfolio, with 10 programs now in either Phase 3 or filed across a number of key therapeutic areas, including regulatory filings for aducanumab in the U.S., the E.U. and Japan. We added or advanced 12 clinical programs in Alzheimer’s disease, MS, ALS, Parkinson’s disease and other movement disorders, depression and biosimilars and had a strong year for business development, including multiple new strategic collaborations. We provided value to our stockholders through the return of approximately $6.7 billion in capital through share repurchases, and we continued our leading efforts in environmental, sustainability and diversity issues. To help ensure the health and safety of our employees, we took several actions in response to the ongoing COVID-19 pandemic. In the U.S. and in most other key markets, our office-based employees began working from home in early March 2020, while we ensured essential staffing levels in our operations remained in place, including maintaining key personnel in our laboratories and manufacturing facilities. To provide a safe work environment for our employees, we have, among other things, increased our cleaning and sanitation routines on our campuses, implemented various social distancing measures on our campuses, created electronic health attestation forms, issued travel advisories to our employees consistent with government regulations and restricted participation of our employees in any events that have large gatherings. We have also suspended the vast majority of our in-person interactions by our customer-facing professionals in healthcare settings and are engaging with these customers remotely as we seek to continue to support healthcare professionals and patient care. Our C&MD Committee considered all of these achievements, and challenges, as they navigated compensation decisions not just for our executive officers but for all of our employees. As described below, our C&MD Committee exercised its discretion and made adjustments to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. Our C&MD Committee believed these adjustments were appropriate because the items were beyond the control of management, were not contemplated and/or could not be quantified due to uncertainty regarding magnitude and timing when the Company performance goals were originally set. Our C&MD Committee also believed that these adjustments were necessary to appropriately motivate and reward employees for their performance during a challenging year in which we continued to perform well despite the challenges that we faced. However, notwithstanding the attainment of our performance goals and the strength of management’s performance, our C&MD Committee also believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals. As a result, our C&MD Committee exercised its discretion and decreased the payouts under certain of our incentive compensation plans for the members of our Executive Committee, including all of our NEOs, as described below. Our C&MD Committee believes that our executive compensation program for 2020 is consistent with our compensation philosophies and principles described below and demonstrates our commitment to linking compensation to Company performance and strategy during a challenging year. | | | | | | | | | | | | |  | | | | Michel Vounatsos
Chief Executive Officer
| | | |  | | | | Susan H. Alexander
Executive Vice President,
Chief Legal Officer and Secretary
| | | | | | | | | | | | | |  | | | | Jeffrey D. Capello
Executive Vice President and
Chief Financial Officer
| | | |  | | | | Paul F. McKenzie, Ph.D.
Executive Vice President,
Pharmaceutical Operations & Technology
| | | | | | | | | | | | | |  | | | | Michael Ehlers, M.D., Ph.D.
Executive Vice President,
Research and Development
| | | | | | | | 33 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
2018 Highlights
We had a productive and successful 2018. We generated record revenues of $13.5 billion for the year, demonstrated resilience in our MS business, continued a strong global launch for SPINRAZA, the first approved treatment for SMA, and made significant progress in our biosimilars business.
We added six clinical programs across our strategic core and emerging growth areas and had a strong year for business development.
We provided value to our stockholders through the return of approximately $4.4 billion in capital through share repurchases and we continued our leading efforts in environmental, sustainability and diversity matters.
Our executive compensation programs for 2018 were aligned with stockholder interests as compensation earned under these programs was closely-linked to the achievement of our corporate performance goals.
We achieved or exceeded the vast majority of the corporate performance goals that we set at the beginning of the year under our incentive compensation plans and, accordingly, the payouts under these plans for 2018 were above target payout levels.
| | | | | 31 | |  | |  |
2020 Highlights | | | 5 | | Executive Compensation Matters (continued)
|
A brief summary of our 2018 business, financial and executive compensation highlights are as follows:
Financial Performance
The following chart provides a summary of our financial performance for 2018 compared to 2017:

A reconciliation of our GAAP toNon-GAAP financial measures is provided in Appendix A to this Proxy Statement.
Total Stockholder Return
Ourone-, three- and five-year total stockholder return (TSR)* compared to our peer group and the Standard & Poor’s 500 (S&P 500) is set forth below.
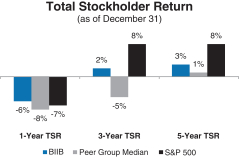
A brief summary of our 2020 business, financial and executive compensation highlights is as follows: Financial Performance The following chart provides a summary of our financial performance for 2020 compared to 2019: * | TSR is a measure of performance over time that combines changes in share price and dividends paid to show the total return to the stockholder expressed as an annualized percentage.
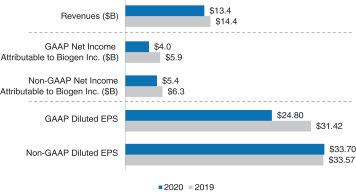
A reconciliation of our GAAP to Non-GAAP financial measures is provided in Appendix A to this Proxy Statement. Product and Pipeline Developments The following provides a summary of our product and pipeline developments for 2020: |
Product and Pipeline Developments
The following provides a summary of our product and pipeline developments for 2018:
Product Developments
In March 2018 we and AbbVie Inc. announced the voluntary worldwide withdrawal of ZINBRYTA for relapsing MS (RMS).
In October 2018 we and Samsung Bioepis launched IMRALDI, an adalimumab biosimilar referencing HUMIRA, in Europe.
Applications for Marketing and Agency Actions Aducanumab In July 2020 we completed the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the approval of aducanumab. In August 2020 the FDA accepted the BLA and granted Priority Review with a Prescription Drug User Fee Act (PDUFA) action date on March 7, 2021. In January 2021 the FDA extended the review period for the BLA for aducanumab by three months. The updated PDUFA action date is June 7, 2021. In October 20182020 the FDA granted BIIB092, ananti-tau mAb, fast track designationEuropean Medicines Agency (EMA) accepted for progressive supranuclear palsy (PSP).review the Marketing Authorization Application (MAA) for aducanumab. In December 2018 Alkermes2020 the Ministry of Health, Labor and Welfare accepted for review the Japanese New Drug Application for aducanumab. SB11 (referencing LUCENTIS) In October 2020 the EMA accepted for review the MAA for SB11, a proposed ranibizumab biosimilar referencing LUCENTIS, and in November 2020 the FDA accepted the BLA for SB11. Ranibizumab is an anti-VEGF (vascular endothelial growth factor) for retinal vascular disorders, which are a leading cause of blindness. MS In March 2020 we made a regulatory submission to the EMA for a subcutaneous (SC) formulation of TYSABRI (natalizumab). In June 2020 we submitted a NDASupplemental Biologics License Application for a SC formulation of natalizumab to the FDAFDA. In October 2020 the first patient in the Phase 1 study of BIIB107 (anti-VLA4) in MS was dosed. In November 2020 we submitted a MAA for VUMERITY (diroximel fumarate; DRF) to the review of BIIB098 (diroximel fumarate). Alkermes is seeking approval of diroximel fumarate under the 505(b)(2) regulatory pathway. If approved, we intend to market diroximel fumarate under the brand name VUMERITY. This name has been conditionally accepted by the FDA and will be confirmed upon approval.EMA. | | | | | | | 32 | | 34 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
Clinical Trials
MS and Neuroimmunology
In September 2018 we completed enrollment of the Phase 2b AFFINITY study evaluating opicinumab, anti-LINGO, as anadd-on therapy in MS patients who are adequately controlled on their anti-inflammatory disease-modifying therapy (DMT), versus the DMT alone.
In November 2018 we initiated the Phase 3b NOVA study evaluating the efficacy and safety of extended interval dosing (every six weeks) for natalizumab compared to standard interval dosing in patients with RMS and enrolled the first patient in December 2018.
In December 2018 we dosed2020 the first patient inEuropean Commission approved a bioequivalence study to test whether exposure levelsnew intramuscular injection route of administration for PLEGRIDY are maintained with intramuscular administration.(peginterferon beta-1a) for the treatment of relapsing-remitting MS. Neuromuscular DisordersClinical Trials
• | | In September 2018 we enrolled the first patient in the Phase 1 study evaluating BIIB078(IONIS-C9Rx), an antisense oligonucleotide (ASO) drug candidate, in adults with C9ORF72-associated ALS.
|
• | | In December 2018 we and our collaboration partner Ionis Pharmaceuticals, Inc. (Ionis) announced results from a positive interim analysis of the ongoing Phase 1 study of BIIB067 (IONIS-SOD1Rx), an investigational treatment for ALS with superoxide dismutase 1 (SOD1) mutations. The interim analysis showed that, over a three-month period, BIIB067 resulted in a statistically significant lowering of SOD1 protein levels in the cerebrospinal fluid and a numerical trend towards slowing of clinical decline as measured by the ALS Functional Rating Scale Revised, both compared to placebo.
|
Alzheimer’s Disease and Dementia In May 2018 we initiatedMarch 2020 the first patient was dosed in the aducanumab re-dosing study, EMBARK, which is a Phase 2global re-dosing clinical study of BIIB092 fordesigned to evaluate aducanumab in eligible Alzheimer’s disease.disease patients who were actively enrolled in aducanumab studies (PRIME, EVOLVE, EMERGE and ENGAGE) in March 2019. In June 2018 we and our collaboration partner Eisai Co., Ltd. (Eisai) announced that elenbecestat,September 2020 the oral BACE (beta amyloid cleaving enzyme) inhibitor, demonstrated an acceptable safety and tolerability profilefirst patient was dosed in the Phase 2 study, and the results demonstrated a statistically significant difference in amyloid-beta levels in the brain measured by3 AHEAD amyloid-PET3-45 (positron emission tomography). A numerical slowing of decline in functional clinical scales of a potentially clinically important difference was also observed, although this effect was not statistically significant. In December 2017 we and our collaboration partner Eisai announced that the Phase 2 study of BAN2401 (lecanemab), an anti-amyloid beta antibody, in individuals with preclinical Alzheimer’s disease who have intermediate or elevated levels of amyloid in their brains. We are collaborating with Eisai on the development of BAN2401.
Neuromuscular Disorders In March 2020 the first patient was dosed in the global DEVOTE study, which is evaluating the safety, tolerability and potential for even greater efficacy of SPINRAZA when administered at a monoclonal antibody that targets amyloid beta aggregates, an Eisai product candidatehigher dose than currently approved for the treatment of Alzheimer’s disease, did not meet the criteria for success based on a Bayesian analysis at 12 months as the primary endpoint in an856-patient Phase 2 clinical study, an endpoint that was designed to enable a potentially more rapid entry into Phase 3 development. In July 2018, based upon the final analysis of the data at 18 months, we and Eisai announced that the topline results from the Phase 2 study demonstrated a statistically significant slowing in clinical decline and reduction of amyloid beta accumulated in the brain. The study achieved statistical significance on key predefined endpoints evaluating efficacy at 18 months on slowing progression in Alzheimer’s Disease Composite Score (ADCOMS) and on reduction of amyloid accumulated in the brain as measured usingamyloid-PET.SMA. In July 2018 we completed enrollmentSeptember 2020 the first patient in a Phase 1 study of ENGAGE and EMERGE, the Phase 3 studies of aducanumab. In March 2019 we and our collaboration partner Eisai announced that we were discontinuing the EMERGE and ENGAGE Phase 3 studies.BIIB105 (ataxin-2 ASO), an antisense oligonucleotide (ASO) targeting ataxin-2 in ALS, was dosed. Movement Disorders In January 2018 we dosedJuly 2020 the first patient in the Phase 2 SPARK1 study of BIIB054,a-synuclein antibody,BIIB101 (ION464), an ASO targeting alpha synuclein in Parkinson’s disease. In September 2018 we completed enrollment of the Phase 2 PASSPORT study of BIIB092 for PSP.multiple system atrophy, was dosed.
Acute NeurologyImmunology
In March 2018 we dosedAugust 2020 the first patient in the Phase 2 OPUS study of natalizumab in drug-resistant focal epilepsy. In September 2018 we enrolled the first patientwas dosed in the Phase 3 CHARM studyprogram for dapirolizumab pegol (anti-CD40L) in patients with active systemic lupus erythematosus despite being treated by standard of BIIB093, glibenclamide IV,care therapies. Dapirolizumab pegol is being developed in large hemispheric infarction, a severe form of ischemic stroke.collaboration with UCB Pharma S.A.
| | | | | 33 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
Neurocognitive DisordersBiosimilars – Samsung Bioepis – Biogen’s Joint Venture with Samsung BioLogics
In December 2018 we dosedMay 2020 Samsung Bioepis announced that the first patientprimary endpoints were met in ourthe randomized, double-masked, Phase 2b study3 trial comparing the efficacy, safety and immunogenicity of BIIB104 (AMPA) in CIAS.SB11 to the reference product (LUCENTIS). Pain
In March 2018 weJune 2020 Samsung Bioepis initiated a Phase 13 study of BIIB095,for SB15, a Nav 1.7 inhibitor for neuropathic pain. In May 2018 we initiated a Phase 2 study of vixotrigine (BIIB074)proposed aflibercept biosimilar referencing EYLEA. EYLEA is widely used to treat ophthalmologic conditions such as neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema (DME) and diabetic retinopathy in small fiber neuropathy.
Other
In September 2018 we dosed the first patient in the Phase 2b study of BG00011(STX-100) in idiopathic pulmonary fibrosis, a chronic irreversible and ultimately fatal disease characterized by a progressive decline in lung function.patients with DME.
Discontinued Programs In February 2018March 2020 we announced that the Phase 2b dose-ranging ACTION2 OPUS study investigating natalizumab as an adjunctive therapy in individualsadults with acute ischemic stroke (AIS)drug-resistant focal epilepsy did not meet its primary endpoint. Safety data were in-line with the known safety profile of natalizumab. Based on these results, we discontinued development of natalizumab in AIS. The results of the Phase 2b ACTION study do not impact the benefit-risk profile of natalizumab in approved indications, including MS.drug-resistant focal epilepsy. In October 20182020 we announced that we completed the Phase 2b2 AFFINITY study of vixotrigine (BIIB074) for the treatment of painful lumbosacral radiculopathy (PLSR). The studyopicinumab (anti-LINGO) in MS did not meet its primary or secondary efficacy endpoints andendpoints. Based on these results, we discontinued development of vixotrigine for the treatment of PLSR. The safety data were consistent with the safety profile reported in previous studies.opicinumab. Business Development In January 2018March 2020 we acquired BIIB100 from Karyopharm Therapeutics Inc. BIIB100 isBIIB118 (CK1 inhibitor), a Phasenovel CNS-penetrant small molecule inhibitor of casein kinase 1, ready investigational oral compound for the potential treatment of certainpatients with behavioral and neurological symptoms across various psychiatric and neurodegenerativeneurological diseases primarily in ALS. BIIB100 is a novel therapeutic candidate that works by inhibiting a protein known as XP01, with the goal of reducing inflammation and neurotoxicity, along with increasing neuroprotective responses. In April 2018 we acquired BIIB104 from Pfizer Inc. BIIB104 is afirst-in-class, Phase 2b ready AMPA receptor potentiatorWe are developing BIIB118 for CIAS, representing our first programthe potential treatment of irregular sleep wake rhythm disorder in neurocognitive disorders. AMPA receptors mediate fast excitatory synaptic transmission in the central nervous system, a process which can be disrupted in a number of neurologicalParkinson’s disease and psychiatric diseases, including schizophrenia.
In June 2018 we closed a10-year exclusive agreement with Ionisplan to develop novel ASO drug candidates for a broad range of neurological diseases (the 2018 Ionis Agreement). We have the option to license therapies arising out of the 2018 Ionis Agreement and will be responsibleBIIB118 for the development and potential commercialization of such therapies.
In June 2018 we entered into an exclusive option agreement with TMS Co., Ltd. granting us the option to acquireTMS-007, a plasminogen activator with a novel mechanism of action associated with breaking down blood clots, which is in Phase 2 development in Japan, and backup compounds for the treatment of stroke.
In June 2018 we exercised our option under our joint venture agreement with Samsung BioLogics to increase our ownership percentagesundowning in Samsung Bioepis from approximately 5% to approximately 49.9%. The share purchase transaction was completed in November 2018.
In July 2018 we acquired BIIB110 (Phase 1a) andALG-802 (preclinical) from AliveGen Inc. BIIB110 andALG-802 represent novel ways of targeting the myostatin pathway. We initially plan to study BIIB110 in multiple neuromuscular indications, including SMA and ALS.
In December 2018 we exercised our option with Ionis and obtained a worldwide, exclusive, royalty-bearing license to develop and commercialize BIIB067, an investigational treatment for ALS with SOD1 mutations.
In December 2018 we entered into a collaborative research and license agreement with C4 Therapeutics (C4T) to investigate the use of C4T’s novel protein degradation platform to discover and develop potential new treatments for neurological diseases, such as Alzheimer’s disease and Parkinson’s disease. We will be responsible for the development and potential commercialization of any therapies resulting from this collaboration.
| | | | | | | 34 | | 35 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
In April 2020 we closed a collaboration and license agreement with Sangamo to develop and commercialize ST-501 for tauopathies, including Alzheimer’s disease; ST-502 for synucleinopathies, including Parkinson’s disease; a third neuromuscular disease target; and up to nine additional neurological disease targets to be identified and selected within a five-year period. The companies are leveraging Sangamo’s proprietary zinc finger protein technology delivered via adeno-associated virus to modulate the expression of key genes involved in neurological diseases. In October 2020 we closed a collaboration and license agreement with Denali to co-develop and co-commercialize Denali’s small molecule inhibitors of leucine-rich repeat kinase 2 (LRRK2) for Parkinson’s disease. In addition to the LRRK2 program, we also have an exclusive option to license two preclinical programs from Denali’s Transport Vehicle platform, including its Antibody Transport Vehicle (ATV): ATV enabled anti-amyloid beta program and a second program utilizing its Transport Vehicle technology. Further, we have a right of first negotiation on two additional Transport Vehicle-enabled therapeutics, should Denali decide to seek a collaboration for such programs. In December 2020 we closed a global collaboration and license agreement with Sage to jointly develop and commercialize BIIB125 (zuranolone) for the potential treatment of major depressive disorder and postpartum depression and BIIB124 (SAGE-324) for the potential treatment of essential tremor with potential in other neurological conditions such as epilepsy. Share Repurchase Activity In August 2018October 2020 our Board of Directors authorized a program to repurchase up to $3.5$5.0 billion of our common stock (2018(2020 Share Repurchase Program). Our 20182020 Share Repurchase Program does not have an expiration date. All share repurchases under our 20182020 Share Repurchase Program will be retired. We returned approximately $4.4$6.7 billion to stockholders in 20182020 through share repurchases under our 20182020 Share Repurchase Program, and our 2016March 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in July 2016March 2019 to repurchase up to $5.0 billion of our common stock and whichthat was completed as of JuneMarch 31, 2020, and our December 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in December 2019 to repurchase up to $5.0 billion of our common stock that was completed as of September 30, 2018.2020. Other Notable Achievements in the Workplace and Community | • | | In September 2020 we announced Healthy Climate, Healthy Lives, a $250.0 million, 20-year initiative to eliminate fossil fuels across our operations and collaborate with renowned institutions with the aim to improve health, especially for the world’s most vulnerable populations. We are the first Fortune 500 company to commit to become fossil fuel free across our operations by 2040. |
Awarded the 2018 International Prix Galien as Best Biotechnology Product for SPINRAZA. The prestigious honor marks the seventh Prix Galien for SPINRAZA, following country recognitionsapproximately €2.4 billion of healthcare savings in the U.S., Germany, Italy, Belgium-Luxembourg, the Netherlands and the U.K. The International Prix Galien is given every two years2020 across Europe that we estimate was contributed by Prix Galien International Committee members in recognition of excellence in scientific innovation to improve human health.our three anti-TNF biosimilars.
Named the Biotechnology Industry Leadernumber one biotechnology company on the Dow Jones Sustainability World Index.Index for the fifth time. Launched our electric vehicle fleet program, expanding our battery electric vehicles to 12 and office chargers to 49 as of December 31, 2020. Recognized as a corporate sustainability leader with the Gold Class and Industry Mover Sustainability AwardsAward from RobecoSAM. Continued commitmentUsed green chemistry processes and techniques to operational carbon neutrality highlighted through the use of 100% renewable electricity globally.reduce our waste and energy consumption.
Committed to reduce carbon emissions by a targeted amount approved byclimate target consistent with reductions required to keep warming to 1.5°C and joined the Science Based Target Initiative,Business Ambition to align ourselves with the global goal of limiting global temperature rise to under two degrees Celsius.1.5°C. Earned CDP scores of A,A-Began engaging our employees and Bsuppliers in the areas of Supplier Engagement, Climate Changetransition to a fossil fuel-free future with 100% renewable electricity targets for suppliers and Water, respectively.sustainable benefit programs for employees.
Earned a perfect score of 100% on the Human Rights Campaign’s Corporate Equality Index (a national benchmarking tool on corporate policies and practices pertinent to LGBTQ employees) for the fifthseventh consecutive year and a perfect score of 100% on the Disability Equality Index for the third consecutive year. Continued our commitment to diversity, equity and inclusion. As of December 31, 2018, 44%2020, 48% of Director-leveldirector-level positions and above were held by women.women, and, in the U.S., 28% were held by ethnic or racial minorities. Over 3,200 employees volunteered from 28 countries during our annual Care Deeply Day.Launched an enhanced strategy with the aim to boost diversity* in U.S. manager positions and above by 30% by the end of 2021.
Engaged 50,000+more than 57,000 students inhands-on learning to inspire their passion for science since the inception of Biogen’s Community Labs.Labs in 2002 with priority focus on underrepresented students. | | | | | | | | | 36 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| * | Percent of U.S. manager positions and above held by Black, African American and Latinx employees as well as Asian employees where underrepresented. |
20182020 Executive Compensation Programs andPay-for-Performance Alignment
We believe our executive compensation programs are effectively designed and have worked well to implement apay-for-performance culture that is aligned with the interests of our stockholders. In 20182020 our executive compensation programs consisted of base salary, short- and long-term incentives and other benefits. 91% of our CEO’s and 84%85% of our other currently-employed NEOs’ 2018(other than our CEO) 2020 target compensation was performance-based andat-risk. 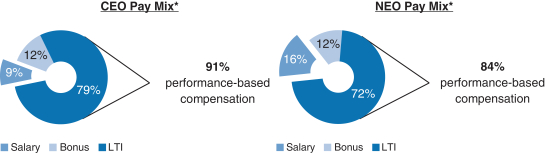 
| | * | Reflects annual salary, target bonus and target grant value of the 20182020 annual long-term incentive awards. The NEO compensation mix excludes theone-time sign-on transitionbonus paid to Mr. McDonnell in connection with his hire, as described in further detail below, as well as compensation for Mr. Capello due to his partial year employment with Biogen in 2020. |
100% of our NEOs’ 2020 annual long-term incentive (LTI) grants were performance-based and at-risk* | | | 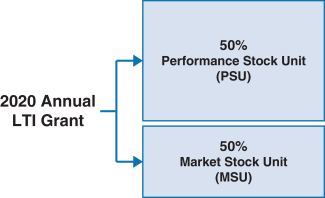
| | • 60% earned based on achievement of three-year pipeline milestone performance goals • 40% earned based on achievement of three one-year financial goals relating to Non-GAAP adjusted free cash flow and revenue • Earned based on stock price performance over one-, two- and three-year periods |
| * | Does not include sign-on LTI awards of RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie,Mr. McDonnell in connection with his hire in August 2020. |
Our 2020 performance-based compensation payouts align with our commitment to strong performance and accountability. Our executive compensation program is structured to closely align with our business purpose and commitment to drive the creation of long-term stockholder value. Our C&MD Committee considered our achievements in 2020 as well as the challenges we faced and made adjustments to certain of the performance goals in our incentive compensation plans to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. At the same time, our C&MD Committee believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals and decreased the payouts under certain of our incentive plans for our NEOs. As a result, the payouts for our NEOs, as a percentage of target, for our 2020 annual bonus plan and the portions of our PSUs and our MSUs that were eligible to be earned based on 2020 performance were below target payout amounts, as described in further detail below. |
| | | | | | | 35 | | 37 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
100% ofWe believe that our NEOs’ 2018 annual long-term incentive (LTI) grants were performance-based andat-risk.
| | | 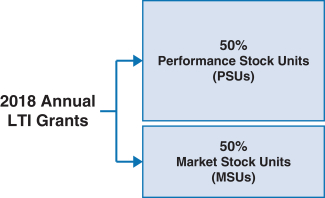 | | • 60% earned based on achievement of three-year adjustedNon-GAAP diluted earnings per share (EPS) and pipeline milestone performance goals
• 40% earned based on achievement of adjustedNon-GAAP free cash flows and revenues over threeone-year performance periods
• PSUs were introduced in 2018. For more information on our PSUs, please see “Long-Term Incentives – 2018 PSUs” below.
• Earned based on stock price performance over one, two and three year periods
|
Our 2018 performance-based2020 executive compensation payouts align withprogram, including the adjustments made by our C&MD Committee, demonstrates our commitment to strong performance.
In 2018 we exceeded the vast majority of the corporatelinking compensation to Company performance goals that we set at the beginning of theand strategy during a challenging year forwhile holding our incentive compensation plans. As a result, the payouts, as a percentage of target, for our 2018 annual bonus plan and the portions of our PSUs and MSUs that were eligible to be earned based on 2018 performance were above target payout amounts, as described in further detail below.executive officers accountable.
20182020 Advisory Vote on Executive Compensation
| | | At our 20182020 annual meeting of stockholders, we continued to receive strong support for our executive compensation programs with approximately 95%83% of the votes cast for approval of our annual“say-on-pay” proposal. Our C&MD Committee viewed this as positive support for our executive compensation programs and their alignment with long-term stockholder value creation and determined that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives. | | 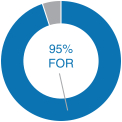
| Our C&MD Committee is committed to continually reviewing our executive compensation programs on a proactive basis to ensure the ongoing alignment of such programs with the interests of our stockholders. | | 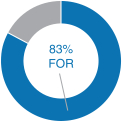 | |
In 20182020 our C&MD Committee reviewed the external landscape,our executive compensation programs in light of market data, the results from our“say-on-pay” proposal at last year’s annual meeting of stockholders and the Company’s performance against the current compensation programs.performance. Our C&MD Committee was satisfied that our existing executive compensation programs further ourpay-for-performance philosophy but made certain enhancementsand, accordingly, did not recommend any significant changes to the design of our LTI program in 2018 to strengthen its focus on long-term performance and alignment with our stockholders’ interests.executive compensation programs for 2020. |
Specifically, under our 2018 LTI program, grants of PSUs replaced grants of cash-settled performance units (CSPUs), which we had granted in previous years. The key changes are as follows:
PSU awards are subject to three-year cliff vesting as compared to annual ratable vesting over three years (1/3 per year) for CSPU awards;
60% of PSU awards are earned over a three-year performance period based on the achievement of three-year cumulative performance goals for stock-settled PSU awards and 40% of PSU awards are earned over three annual performance periods based on the achievement of three sets of annual performance goals for cash-settled PSU awards as compared to 100% of CSPUs awards earned based upon one annual performance period for CSPU awards; and
60% of the PSU awards will be settled in stock and 40% of the PSU awards will be settled in cash as compared to 100% cash settlement for CSPU awards.
| | | | | 36 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
For additional information on our PSU awards, please see “Long-Term Incentives – 2018 PSUs” below.
Roles and Responsibilities Role of our C&MD Committee Our C&MD Committee, which is composed of fourthree independent directors, oversees and administers our executive compensation programs. In making executive compensation decisions, our C&MD Committee reviews a variety of factors and data, most importantly our performance and individual executives’executive performance, and considers the totality of compensation that may be paid.paid or the value of which that may be granted. In addition, our C&MD Committee administers our annual bonus plan and our equity plans, reviews business achievements relevant to payouts under our compensation plans, makes recommendations to our Board of Directors with respect to compensation policies and practices as well as the compensation of our CEO and seeks to ensure that total compensation paid to our executive officers is fair, competitive and aligned with stockholder interests. Our C&MD Committee retains the right to hire outside advisors as needed to assist it in reviewing and revising our executive compensation programs. The duties and responsibilities of our C&MD Committee are described on page 2019 and can be found in our C&MD Committee’s written charter adopted by our Board of Directors, which can be found on our website,www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. RoleMichel Vounatsos
| | | | 53,072 | | | | | — | | | | | 53,072 | | | | | * | | Michael R. McDonnell | | | | — | | | | | — | | | | | — | | | | | — | | Alfred W. Sandrock, Jr. | | | | 17,841 | | | | | — | | | | | 17,841 | | | | | * | | Susan H. Alexander | | | | 41,576 | | | | | — | | | | | 41,576 | | | | | * | | Chirfi Guindo | | | | 6,006 | | | | | — | | | | | 6,006 | | | | | * | | Jeffrey D. Capello(7) | | | | 3,118 | | | | | — | | | | | 3,118 | | | | | * | | Directors | | | | | | | | | | | | | | | | | | | | | Alexander J. Denner(8) | | | | 655,064 | | | | | 890 | | | | | 655,954 | | | | | * | | Caroline D. Dorsa | | | | 20,207 | | | | | 890 | | | | | 21,097 | | | | | * | | Maria C. Freire | | | | — | | | | | — | | | | | — | | | | | — | | William A. Hawkins | | | | 1,115 | | | | | 890 | | | | | 2,045 | | | | | * | | William D. Jones | | | | — | | | | | — | | | | | — | | | | | — | | Nancy L. Leaming | | | | 12,098 | | | | | 890 | | | | | 12,988 | | | | | * | | Jesus B. Mantas | | | | 2,053 | | | | | 890 | | | | | 2,943 | | | | | * | | Richard C. Mulligan | | | | 12,064 | | | | | 890 | | | | | 12,954 | | | | | * | | Robert W. Pangia(9) | | | | 19,742 | | | | | 890 | | | | | 20,632 | | | | | * | | Stelios Papadopoulos(10) | | | | 33,301 | | | | | 1,470 | | | | | 34,771 | | | | | * | | Brian S. Posner | | | | 6,870 | | | | | 890 | | | | | 7,760 | | | | | * | | Eric K. Rowinsky | | | | 16,179 | | | | | 890 | | | | | 17,069 | | | | | * | | Stephen A. Sherwin | | | | 15,438 | | | | | 890 | | | | | 16,328 | | | | | * | | Executive officers and directors as a group (21 persons)(11) | | | | 926,695 | | | | | 10,370 | | | | | 937,065 | | | | | * | |
| * | Represents beneficial ownership of less than 1% of our outstanding shares of common stock. |
| | | | | | | | | 26 | | 
| |  |
| | | | 4 | | Stock Ownership (continued) |
| (1) | The shares described as “owned” are shares of our common stock directly or indirectly owned by each listed person, rounded up to the nearest whole share. |
| (2) | Includes RSUs that will vest within 60 days of the Independent Compensation ConsultantOwnership Date. |
| (3) | Our C&MD Committee believes that independent adviceThe calculation of percentages is important in developing and overseeing our executive compensation programs. Frederic W. Cook & Co., Inc. (FW Cook) served as our C&MD Committee’s independent compensation consultant until June 2018 and advised our C&MD Committee regarding compensation decisions in 2018. FW Cook did not provide any other services to Biogen. Pearl Meyer & Partners LLC (Pearl Meyer) has served as our C&MD Committee’s independent compensation consultant since June 2018 and has advised our C&MD Committee regarding compensation decisions since that time. Pearl Meyer does not provide any other services to Biogen and engages in other matters as needed and as directed solely by our C&MD Committee. References in this CD&A to our independent compensation consultant refer to FW Cookbased upon 150,554,556 shares outstanding on the Ownership Date, plus for the period during which it was engaged and to Pearl Meyer thereafter.
Reporting directly to our C&MD Committee, our independent compensation consultant provides guidance on trends in CEO, executive andnon-employee director compensation, the development of specific executive compensation programs and the compositioneach of the Company’s compensation peer group. Additionally,individuals listed above the shares subject to RSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and Stock Units.”
|
| (4) | Based solely on information as of December 31, 2020, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 12, 2021, which also indicates that it has sole voting power over 15,443,182 shares and sole dispositive power over 15,822,066 shares. |
| (5) | Based solely on information as of December 31, 2020, contained in a Schedule 13G/A filed with the SEC by BlackRock, Inc. on January 29, 2021, which also indicates that it has sole voting power with respect to 11,672,922 shares and sole dispositive power with respect to 13,419,601 shares. |
| (6) | Based solely on information as of December 31, 2020, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 10, 2021, which also indicates that it has sole dispositive power with respect to 11,213,323 shares, shared voting power with respect to 259,934 shares and shared dispositive power with respect to 638,187 shares. |
| (7) | Mr. Capello ceased to be our independent compensation consultant preparesExecutive Vice President and Chief Financial Officer on August 15, 2020, and separated from the Company on September 15, 2020. |
| (8) | Includes 643,000 shares beneficially owned by funds and accounts managed by Sarissa Capital Management LP, a report on CEO payDelaware limited partnership (Sarissa Capital). Dr. Denner is the Chief Investment Officer of Sarissa Capital and ultimately controls the funds and accounts managed by Sarissa Capital. By virtue of the foregoing, Dr. Denner may be deemed to indirectly beneficially own (as that compares each elementterm is defined in Rule 13d-3 of compensationthe Exchange Act) the 643,000 shares that those entities beneficially own. Dr. Denner disclaims beneficial ownership of these shares except to thatthe extent of CEOs in comparable positions at companies in our peer group. Using this and other similar information, our C&MD Committee recommends, andany pecuniary interest therein. |
| (9) | Includes 16,000 shares beneficially owned by Robin Drive, LLC, of which Mr. Pangia’s wife is the Trustee. Mr. Pangia is retiring from our Board of Directors, approves, the elements and target levels of our CEO’s compensation. During 2018 the Company paid FW Cook and Pearl Meyer $123,275 and $47,666, respectively, in consulting fees directly related to these services. Our C&MD Committee assessed FW Cook’s independence annually and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2017 that FW Cook’s work did not raise any conflicts of interest and that FW Cook remained independent under applicable rules. Our C&MD Committee assessed Pearl Meyer’s independence in connection with its engagement in June 2018 and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2018 that Pearl Meyer’s work did not raise any conflicts of interest and that Pearl Meyer remains independent under applicable rules.
Role of our CEO
Each year our CEO provides an assessmenteffective as of the performanceAnnual Meeting.
|
| (10) | Includes 28,206 shares held in limited liability companies of each executive officer, other than himself, duringwhich Dr. Papadopoulos is the prior year and recommends to our C&MD Committee the compensation to be paidsole manager. |
| (11) | Includes 688,175 shares held indirectly through trusts, funds, defined benefit plans or awarded to each executive. Our CEO’s recommendations are based on numerous factors, including:limited liability companies. |
Company, team and individual performance;
potential for future contributions;
leadership competencies;
external market competitiveness;
internal pay comparisons; and
other factors deemed relevant.
| | | | | | | | | To understand the external market competitiveness of the compensation for our executive officers, our CEO and our C&MD Committee review a report analyzing publicly-available information and surveys prepared by our internal27
| | 
| | | | | 37 | |  | |  |
| | | | | | | Proposal 2 – Ratification of the Selection of Our Independent Registered Public Accounting Firm | | | | | | | |
Our Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the independent registered public accounting firm retained to audit our consolidated financial statements. Our Audit Committee has selected PwC as our independent registered public accounting firm for the fiscal year ending December 31, 2021. PwC has served as our independent registered public accounting firm since 2003. In order to assure continuing auditor independence, our Audit Committee periodically considers whether there should be a rotation of the independent registered public accounting firm. Further, in conjunction with the rotation of the auditing firm’s lead engagement partner required by applicable SEC rules, our Audit Committee and its Chair has in the past been, and in the future will be, directly involved in the selection of PwC’s new lead engagement partner. Our Audit Committee believes at this time that the continued retention of PwC to serve as our independent registered public accounting firm is in the best interest of Biogen and its stockholders. Although stockholder approval of our Audit Committee’s selection of PwC is not required, our Board of Directors believes that it is a matter of good corporate practice to solicit stockholder ratification of this selection. If our stockholders do not ratify the selection of PwC as our independent registered public accounting firm, our Audit Committee will reconsider its selection. Even if the selection is ratified, our Audit Committee always has the ability to change the engagement of PwC if it considers that a change is in Biogen’s best interest. Representatives of PwC will participate in the Annual Meeting, have the opportunity to make a statement if they so desire and be available to respond to appropriate questions. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE RATIFICATION OF THE SELECTION OF PRICEWATERHOUSECOOPERS LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR THE FISCAL YEAR ENDING DECEMBER 31, 2021. | | | | | | | | | 28 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit Committee Report The Audit Committee’s role is to act on behalf of our Board of Directors in the oversight of Biogen’s financial reporting, internal control and audit functions. The roles and responsibilities of the Audit Committee are set forth in the written charter adopted by our Board of Directors, which is posted on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. Management has primary responsibility for the financial statements and the reporting process, including the systems of internal control. In fulfilling its oversight responsibilities, the Audit Committee, among other things: reviewed and discussed with management the audited consolidated financial statements contained in Biogen’s 2020 Annual Report on Form 10-K; discussed with PwC, Biogen’s independent registered public accounting firm, the overall scope and plans for the audit; met with PwC, with and without management present, to discuss the results of its examination, management’s response to any significant findings, its observations of Biogen’s internal control, the overall quality of Biogen’s financial reporting, the selection, application and disclosure of critical accounting policies, new accounting developments and accounting-related disclosures, the key accounting judgments and assumptions made in preparing the financial statements and whether the financial statements would have materially changed had different judgments and assumptions been made and other pertinent items related to Biogen’s accounting, internal control and financial reporting; discussed with representatives of Biogen’s corporate internal audit staff, with and without management present, their purpose, authority, audit plan and reports; reviewed and discussed with PwC the matters required by the Public Company Accounting Oversight Board and the SEC; discussed with PwC its independence from management and Biogen, including the written disclosures and letter concerning independence received from PwC under applicable requirements of the Public Company Accounting Oversight Board. The Audit Committee has determined that the provision of non-audit services to Biogen by PwC is compatible with its independence; provided oversight and advice to management in connection with Biogen’s system of internal control over financial reporting in response to the requirements set forth in Section 404 of the Sarbanes-Oxley Act of 2002 and related regulations. In connection with this oversight, the Audit Committee reviewed a report by management on the effectiveness of Biogen’s internal control over financial reporting; and reviewed PwC’s Report of Independent Registered Public Accounting Firm included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, related to its audit of the effectiveness of internal control over financial reporting. In reliance on these reviews and discussions, the Audit Committee recommended to our Board of Directors that the audited consolidated financial statements be included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, for filing with the SEC. The Audit Committee of our Board of Directors: Caroline D. Dorsa (Chair) William A. Hawkins Nancy L. Leaming Stephen A. Sherwin, M.D. | | | | | | | | | 29 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit and Other Fees The following table shows fees for professional audit services billed to us by PwC for the audit of our annual consolidated financial statements for the years ended December 31, 2020 and December 31, 2019, and fees billed to us by PwC for other services provided during 2020 and 2019: | | | | | | | | | | | | Fees (amounts in thousands) | | 2020 | | | 2019 | | Audit fees | | $ | 5,882.0 | | | $ | 6,080.3 | | Audit-related fees | | | 48.2 | | | | 55.0 | | Tax fees* | | | 392.5 | | | | 641.8 | | All other fees | | | 9.9 | | | | 7.0 | | Total | | $ | 6,332.6 | | | $ | 6,784.1 | |
| * | Includes tax compliance fees of approximately $0.1 million in 2020 and 2019. |
Audit fees are fees for the audit of our 2020 and 2019 consolidated financial statements included in our Annual Reports on Form 10-K, reviews of our condensed consolidated financial statements included in our Quarterly Reports on Form 10-Q, review of the consolidated financial statements incorporated by reference into our outstanding registration statements and statutory audit fees in overseas jurisdictions. Audit-related fees are fees that principally relate to assurance and related services that are also performed by our independent registered public accounting firm. More specifically, these services include audits of employee benefit plan information, accounting consultations, due diligence and audits in connection with business development activity, internal control reviews and attest services related to financial reporting that are not required by statute or regulation. Tax fees are fees for tax compliance and planning services. All other fees in 2020 and 2019 also includelicense fees for a web-based accounting research tool. Policy on Pre-Approval of Audit and Non-Audit Services Our Audit Committee has the sole authority to approve the scope of the audit and any audit-related services as well as all audit fees and terms. Our Audit Committee must pre-approve any audit and non-audit services provided by our independent registered public accounting firm. Our Audit Committee will not approve the engagement of the independent registered public accounting firm to perform any services that the independent registered public accounting firm would be prohibited from providing under applicable securities laws, Nasdaq requirements or Public Company Accounting Oversight Board rules. In assessing whether to approve the use of our independent registered public accounting firm to provide permitted non-audit services, our Audit Committee tries to minimize relationships that could appear to impair the objectivity of our independent registered public accounting firm. Our Audit Committee will approve permitted non-audit services by our independent registered public accounting firm only when it will be more effective or economical to have such services provided by our independent registered public accounting firm than by another firm. Our Audit Committee annually reviews and pre-approves the audit, audit-related, tax and other permissible non-audit services that can be provided by the independent registered public accounting firm. After the annual review, any proposed services exceeding pre-set levels or amounts, or additional services not previously approved requires separate pre-approval by our Audit Committee or the Chair of our Audit Committee. Any pre-approval decision made by the Chair of our Audit Committee is reported to our Audit Committee at the next regularly scheduled Audit Committee meeting. Our Chief Financial Officer and our Chief Accounting Officer can approve up to an additional $50,000 in the aggregate per calendar year for categories of services that our Audit Committee (or the Chair through its delegated authority) has pre-approved. All of the services provided by PwC during 2020 and 2019 were pre-approved in accordance with this policy. | | | | | | | | | 30 | | 
| |  |
| | | | 6 | | Executive Compensation Matters |
| | | | | | | | | | | | Proposal 3 – Advisory Vote on Executive Compensation | | | | | | | |
Our Compensation Discussion and Analysis, which appears below, describes our executive compensation programs and the compensation decisions that our C&MD Committee and our Board of Directors made with respect to the 2020 compensation of our named executive officers. As required pursuant to Section 14A of the Exchange Act, our Board of Directors is asking that stockholders cast a non-binding, advisory vote FOR the following resolution: “RESOLVED, that the compensation paid to the Company’s named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.” Our Board of Directors is asking that our stockholders support this proposal. Although the vote you are being asked to cast is non-binding, we value the views of our stockholders, and our C&MD Committee and our Board of Directors will consider the outcome of the vote when making future compensation decisions for our named executive officers. As we describe in our Compensation Discussion and Analysis, our executive compensation programs embody a pay-for-performance philosophy that supports our business strategy and aligns the interests of our executives with those of our stockholders. In particular, our executive compensation programs reward financial, strategic and operational performance, and the goals set under our plans support our short- and long-range plans. In addition, to discourage excessive risk taking, we maintain policies for stock ownership, and our equity and annual bonus incentive plans have provisions providing for the recoupment of compensation. We also cap payments under our annual bonus plan, and we generally require multi-year vesting periods for long-term incentive awards. We will hold a non-binding, advisory vote of our stockholders on the compensation of our named executive officers every year until the next required stockholder vote on the frequency of such advisory vote. The next stockholder vote on the frequency of such advisory vote is expected to be held at the 2023 annual meeting of stockholders. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE APPROVAL OF THE RESOLUTION SET FORTH ABOVE. | | | | | | | | | 31 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| compensation group and reviewed by our independent compensation consultant. The report compares the compensation of each executive officer, other than our CEO, to data for comparable positions at companies in our peer group, by compensation element (please see “External Market Competitiveness and Peer Group” below for further details). Our C&MD Committee considers all of the information presented, discusses the recommendations with our CEO and with our independent compensation consultant and applies its judgment to determine the elements of compensation and target compensation levels for each executive officer other than the CEO.
Our CEO also provides a self-assessment of his achievements for the prior year. Our C&MD Committee reviews and considers this in analyzing the CEO’s performance, and in recommending for approval by our Board of Directors, the compensation of our CEO. Our CEO does not participate in any deliberations regarding his own compensation.
Executive Compensation Philosophy and Objectives COMPENSATION DISCUSSION AND ANALYSIS
Our executive compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation that is competitive with our peer group in order to attract and retain extraordinary leaders who can perform at high levels and succeed in a demanding business environment. We aim to achieve this by designing programs that are:
• | | Mission Focused and Business Driven. Our executive compensation programs support the relentless pursuit of delivering meaningful and innovative therapies to patients by providing our executives with incentives to achieve the near- and long-term objectives of our business. Substantially all of our executive incentive compensation programs are tied directly, and meaningfully, to Company performance. Our objective is to emphasize the importance of achieving short-term goals while building and sustaining a foundation for long-term success. |
• | | Competitively Advantageous. We benchmark our executive compensation programs against a peer group of biotechnology and pharmaceutical companies that we believe are representative of the companies we primarily compete with for talent, balanced with factors such as business scope and size, including revenues and market capitalization, business focus and geographic scope of operations. Peer group practices are among the many factors we take into account in developing compensation programs that we believe are most effective, and which |
| | enable us to recruit, retain and motivate our leadership team to achieve their best for Biogen and our stockholders.
|
This Compensation Discussion and Analysis (CD&A) describes our compensation strategy, philosophy, policies and practices underlying our executive compensation programs for 2020. It also provides information regarding the compensation that was earned by and awarded to our 2020 named executive officers, whom we refer to collectively as “named executive officers” or “NEOs.” Our named executive officers include our current executive officers listed below as well as Jeffrey D. Capello*, our former Executive Vice President and Chief Financial Officer. | | | | | | | | | | | | |  | | | | Michel Vounatsos Chief Executive Officer | | | |  | | | | Susan H. Alexander Executive Vice President, Chief Legal Officer and Secretary | | | | | | | | | | | | | |  | | | | Michael R. McDonnell* Executive Vice President and Chief Financial Officer | | | |  | | | | Chirfi Guindo Executive Vice President, Global Product Strategy and Commercialization | | | | | | | | | | | | | |  | | | | Alfred W. Sandrock, Jr., M.D., Ph.D.** Executive Vice President, Research and Development | | | | | | • | | Performance Differentiated. We believe strongly inpay-for-performance and endeavor to significantly differentiate rewards by delivering the highest rewards to our best performers and lesser rewards to those who do not meet our performance expectations. |
• | | Ownership Aligned. At Biogen, we believe every employee contributes to the success of the Company and, as such, every employee has a vested interest in the Company’s success. To reinforce this alignment with our stockholders, we strongly encourage stock ownership through our equity-based compensation programs. For members of our executive team, including our NEOs, who set and lead the future strategic direction of our Company, we ensure that a significant portion of their total pay opportunities are equity-based to maintain alignment between the interests of our executive officers and our stockholders.| * | Mr. McDonnell was appointed as Executive Vice President and Chief Financial Officer effective August 15, 2020. Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer on August 15, 2020, and separated from the Company on September 15, 2020. |
• | | Flexible. We are committed to providing flexible benefits designed to allow our diverse global workforce to have reward opportunities that meet their varied needs so that they are inspired to perform their very best on behalf of patients and stockholders each day.| ** | Dr. Sandrock was appointed as Executive Vice President, Research and Development on October 1, 2019. Prior to this appointment, Dr. Sandrock served as our Executive Vice President, Chief Medical Officer, and continued in this role, in addition to his duties as Executive Vice President, Research and Development, until January 27, 2020. |
| External Market Competitiveness Executive Summary
|
We had a productive and successful 2020 as we continued to execute well against our corporate strategy. Our full year revenue for 2020 was $13.4 billion, a 6% decrease from the prior year primarily due to the entry of multiple TECFIDERA generic entrants in the U.S. with deeply discounted prices compared to TECFIDERA. The generic competition for TECFIDERA significantly reduced our TECFIDERA revenues during the year ended December 31, 2020, and is expected to have a substantial negative impact on our TECFIDERA revenues for as long as there is generic competition. Excluding TECFIDERA in the U.S., our global MS revenue, including OCREVUS royalties, remained relatively stable for 2020, as compared to 2019, demonstrating the resilience of our MS business in a competitive market. We continued our launch of VUMERITY in the U.S., which was the number two MS product and the number one oral in terms of new prescriptions in the U.S. as of December 31, 2020. We maintained our leadership in our SMA business despite increased competition and delays in SPINRAZA doses due, directly or indirectly, to the COVID-19 pandemic. Although our full year 2020 SPINRAZA revenue decreased 2% as compared to 2019, we continued to see growth outside of the U.S., with full year 2020 revenue outside the U.S. growing 9% as compared to 2019, and we believe that SPINRAZA will remain a foundation of care in the treatment of SMA. | | | | | | | | | 32 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Our full year 2020 biosimilars revenue increased 8% as compared to 2019. Although our biosimilars business was negatively impacted by pricing pressure and a slowdown in new treatments and reduced clinic capacity due to the COVID-19 pandemic, we were the leading anti-TNF biosimilar provider in Europe in 2020, and BENEPALI was the #1 prescribed etanercept product across Europe. We also made significant progress toward building a multi-franchise portfolio, with 10 programs now in either Phase 3 or filed across a number of key therapeutic areas, including regulatory filings for aducanumab in the U.S., the E.U. and Japan. We added or advanced 12 clinical programs in Alzheimer’s disease, MS, ALS, Parkinson’s disease and other movement disorders, depression and biosimilars and had a strong year for business development, including multiple new strategic collaborations. We provided value to our stockholders through the return of approximately $6.7 billion in capital through share repurchases, and we continued our leading efforts in environmental, sustainability and diversity issues. To help ensure the health and safety of our employees, we took several actions in response to the ongoing COVID-19 pandemic. In the U.S. and in most other key markets, our office-based employees began working from home in early March 2020, while we ensured essential staffing levels in our operations remained in place, including maintaining key personnel in our laboratories and manufacturing facilities. To provide a safe work environment for our employees, we have, among other things, increased our cleaning and sanitation routines on our campuses, implemented various social distancing measures on our campuses, created electronic health attestation forms, issued travel advisories to our employees consistent with government regulations and restricted participation of our employees in any events that have large gatherings. We have also suspended the vast majority of our in-person interactions by our customer-facing professionals in healthcare settings and are engaging with these customers remotely as we seek to continue to support healthcare professionals and patient care. Our C&MD Committee considered all of these achievements, and challenges, as they navigated compensation decisions not just for our executive officers but for all of our employees. As described below, our C&MD Committee exercised its discretion and made adjustments to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. Our C&MD Committee believed these adjustments were appropriate because the items were beyond the control of management, were not contemplated and/or could not be quantified due to uncertainty regarding magnitude and timing when the Company performance goals were originally set. Our C&MD Committee also believed that these adjustments were necessary to appropriately motivate and reward employees for their performance during a challenging year in which we continued to perform well despite the challenges that we faced. However, notwithstanding the attainment of our performance goals and the strength of management’s performance, our C&MD Committee also believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals. As a result, our C&MD Committee exercised its discretion and decreased the payouts under certain of our incentive compensation plans for the members of our Executive Committee, including all of our NEOs, as described below. Our C&MD Committee believes that our executive compensation program for 2020 is consistent with our compensation philosophies and principles described below and demonstrates our commitment to linking compensation to Company performance and strategy during a challenging year. | | | | | | | | | 33 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
2020 Highlights A brief summary of our 2020 business, financial and executive compensation highlights is as follows: Financial Performance The following chart provides a summary of our financial performance for 2020 compared to 2019: 
A reconciliation of our GAAP to Non-GAAP financial measures is provided in Appendix A to this Proxy Statement. Product and Pipeline Developments The following provides a summary of our product and pipeline developments for 2020: Applications for Marketing and Agency Actions Aducanumab In July 2020 we completed the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the approval of aducanumab. In August 2020 the FDA accepted the BLA and granted Priority Review with a Prescription Drug User Fee Act (PDUFA) action date on March 7, 2021. In January 2021 the FDA extended the review period for the BLA for aducanumab by three months. The updated PDUFA action date is June 7, 2021. In October 2020 the European Medicines Agency (EMA) accepted for review the Marketing Authorization Application (MAA) for aducanumab. In December 2020 the Ministry of Health, Labor and Welfare accepted for review the Japanese New Drug Application for aducanumab. SB11 (referencing LUCENTIS) In October 2020 the EMA accepted for review the MAA for SB11, a proposed ranibizumab biosimilar referencing LUCENTIS, and in November 2020 the FDA accepted the BLA for SB11. Ranibizumab is an anti-VEGF (vascular endothelial growth factor) for retinal vascular disorders, which are a leading cause of blindness. MS In March 2020 we made a regulatory submission to the EMA for a subcutaneous (SC) formulation of TYSABRI (natalizumab). In June 2020 we submitted a Supplemental Biologics License Application for a SC formulation of natalizumab to the FDA. In October 2020 the first patient in the Phase 1 study of BIIB107 (anti-VLA4) in MS was dosed. In November 2020 we submitted a MAA for VUMERITY (diroximel fumarate; DRF) to the EMA. | | | | | | | | | 34 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
In December 2020 the European Commission approved a new intramuscular injection route of administration for PLEGRIDY (peginterferon beta-1a) for the treatment of relapsing-remitting MS. Clinical Trials Alzheimer’s Disease and Dementia In March 2020 the first patient was dosed in the aducanumab re-dosing study, EMBARK, which is a global re-dosing clinical study designed to evaluate aducanumab in eligible Alzheimer’s disease patients who were actively enrolled in aducanumab studies (PRIME, EVOLVE, EMERGE and ENGAGE) in March 2019. In September 2020 the first patient was dosed in the Phase 3 AHEAD 3-45 clinical study of BAN2401 (lecanemab), an anti-amyloid beta antibody, in individuals with preclinical Alzheimer’s disease who have intermediate or elevated levels of amyloid in their brains. We are collaborating with Eisai on the development of BAN2401. Neuromuscular Disorders In March 2020 the first patient was dosed in the global DEVOTE study, which is evaluating the safety, tolerability and potential for even greater efficacy of SPINRAZA when administered at a higher dose than currently approved for the treatment of SMA. In September 2020 the first patient in a Phase 1 study of BIIB105 (ataxin-2 ASO), an antisense oligonucleotide (ASO) targeting ataxin-2 in ALS, was dosed. Movement Disorders In July 2020 the first patient in the Phase 1 study of BIIB101 (ION464), an ASO targeting alpha synuclein in multiple system atrophy, was dosed. Immunology In August 2020 the first patient was dosed in the Phase 3 program for dapirolizumab pegol (anti-CD40L) in patients with active systemic lupus erythematosus despite being treated by standard of care therapies. Dapirolizumab pegol is being developed in collaboration with UCB Pharma S.A. Biosimilars – Samsung Bioepis – Biogen’s Joint Venture with Samsung BioLogics In May 2020 Samsung Bioepis announced that the primary endpoints were met in the randomized, double-masked, Phase 3 trial comparing the efficacy, safety and immunogenicity of SB11 to the reference product (LUCENTIS). In June 2020 Samsung Bioepis initiated a Phase 3 study for SB15, a proposed aflibercept biosimilar referencing EYLEA. EYLEA is widely used to treat ophthalmologic conditions such as neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema (DME) and diabetic retinopathy in patients with DME. Discontinued Programs In March 2020 we announced that the Phase 2 OPUS study investigating natalizumab as an adjunctive therapy in adults with drug-resistant focal epilepsy did not meet its primary endpoint. Safety data were in-line with the known safety profile of natalizumab. Based on these results, we discontinued development of natalizumab in drug-resistant focal epilepsy. In October 2020 we announced that the Phase 2 AFFINITY study of opicinumab (anti-LINGO) in MS did not meet its primary or secondary endpoints. Based on these results, we discontinued development of opicinumab. Business Development In March 2020 we acquired BIIB118 (CK1 inhibitor), a novel CNS-penetrant small molecule inhibitor of casein kinase 1, for the potential treatment of patients with behavioral and neurological symptoms across various psychiatric and neurological diseases from Pfizer Inc. We are developing BIIB118 for the potential treatment of irregular sleep wake rhythm disorder in Parkinson’s disease and plan to develop BIIB118 for the potential treatment of sundowning in Alzheimer’s disease. | | | | | | | | | 35 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
In April 2020 we closed a collaboration and license agreement with Sangamo to develop and commercialize ST-501 for tauopathies, including Alzheimer’s disease; ST-502 for synucleinopathies, including Parkinson’s disease; a third neuromuscular disease target; and up to nine additional neurological disease targets to be identified and selected within a five-year period. The companies are leveraging Sangamo’s proprietary zinc finger protein technology delivered via adeno-associated virus to modulate the expression of key genes involved in neurological diseases. In October 2020 we closed a collaboration and license agreement with Denali to co-develop and co-commercialize Denali’s small molecule inhibitors of leucine-rich repeat kinase 2 (LRRK2) for Parkinson’s disease. In addition to the LRRK2 program, we also have an exclusive option to license two preclinical programs from Denali’s Transport Vehicle platform, including its Antibody Transport Vehicle (ATV): ATV enabled anti-amyloid beta program and a second program utilizing its Transport Vehicle technology. Further, we have a right of first negotiation on two additional Transport Vehicle-enabled therapeutics, should Denali decide to seek a collaboration for such programs. In December 2020 we closed a global collaboration and license agreement with Sage to jointly develop and commercialize BIIB125 (zuranolone) for the potential treatment of major depressive disorder and postpartum depression and BIIB124 (SAGE-324) for the potential treatment of essential tremor with potential in other neurological conditions such as epilepsy. Share Repurchase Activity In October 2020 our Board of Directors authorized a program to repurchase up to $5.0 billion of our common stock (2020 Share Repurchase Program). Our 2020 Share Repurchase Program does not have an expiration date. All share repurchases under our 2020 Share Repurchase Program will be retired. We returned approximately $6.7 billion to stockholders in 2020 through share repurchases under our 2020 Share Repurchase Program, our March 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in March 2019 to repurchase up to $5.0 billion of our common stock that was completed as of March 31, 2020, and our December 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in December 2019 to repurchase up to $5.0 billion of our common stock that was completed as of September 30, 2020. Other Notable Achievements in the Workplace and Community | • | | In September 2020 we announced Healthy Climate, Healthy Lives, a $250.0 million, 20-year initiative to eliminate fossil fuels across our operations and Peer Group collaborate with renowned institutions with the aim to improve health, especially for the world’s most vulnerable populations. We consider market practices and trends when determining executive compensation levels and compensation program designs at Biogen. We do not target a specific market percentile or simply replicateare the market practice. Instead, we review external market practices as a reference pointfirst Fortune 500 company to assist us in providing programs designedcommit to attract, retain and inspire extraordinary talent. Our C&MD Committee also uses a peer group to provide context for its executive compensation decision-making. Each yearbecome fossil fuel free across our independent compensation consultant reviews the external market landscape and evaluates the composition of our peer group for appropriateness. Our C&MD Committee reviews the information provided from internal sources as well as the information providedoperations by our independent compensation consultant to select our peer group based on comparable companies that approximate (1) our scope of business, including revenues and market capitalization, (2) our global geographical reach, (3) our research-based business with multiple marketed products and (4) a comparable pool of talent for which we compete.2040.
The peer group for determining our 2018 compensation decisions consisted of biotechnology and pharmaceutical
The approximately €2.4 billion of healthcare savings in 2020 across Europe that we estimate was contributed by our three anti-TNF biosimilars. Named the number one biotechnology company on the Dow Jones Sustainability World Index for the fifth time. Launched our electric vehicle fleet program, expanding our battery electric vehicles to 12 and office chargers to 49 as of December 31, 2020. Recognized as a corporate sustainability leader with the Gold Class Sustainability Award from RobecoSAM. Used green chemistry processes and techniques to reduce our waste and energy consumption. Committed to a climate target consistent with reductions required to keep warming to 1.5°C and joined the Business Ambition to 1.5°C. Began engaging our employees and suppliers in the transition to a fossil fuel-free future with 100% renewable electricity targets for suppliers and sustainable benefit programs for employees. Earned a perfect score of 100% on the Human Rights Campaign’s Corporate Equality Index (a national benchmarking tool on corporate policies and practices pertinent to LGBTQ employees) for the seventh consecutive year and a perfect score of 100% on the Disability Equality Index for the third consecutive year. Continued our commitment to diversity, equity and inclusion. As of December 31, 2020, 48% of director-level positions and above were held by women, and, in the U.S., 28% were held by ethnic or racial minorities. Launched an enhanced strategy with the aim to boost diversity* in U.S. manager positions and above by 30% by the end of 2021. Engaged more than 57,000 students in hands-on learning to inspire their passion for science since the inception of Biogen’s Community Labs in 2002 with priority focus on underrepresented students. | | | | | 38 | |  | |  |
| | | 36 | | 
| |  | 5 |
| | | | 6 | | Executive Compensation Matters (continued) |
| * | companies,Percent of U.S. manager positions and above held by Black, African American and Latinx employees as we compete with companies in both of these sectors for executive talent.well as Asian employees where underrepresented.
|
2020 Executive Compensation Programs and Pay-for-Performance Alignment We believe our executive compensation programs are effectively designed and have worked well to implement a pay-for-performance culture that is aligned with the interests of our stockholders. In 2020 our executive compensation programs consisted of base salary, short- and long-term incentives and other benefits. 91% of our CEO’s and 85% of our other currently-employed NEOs’ (other than our CEO) 2020 target compensation was performance-based and at-risk. 
| * | Reflects annual salary, target bonus and target grant value of the 2020 annual long-term incentive awards. The NEO compensation mix excludes the one-time sign-on bonus paid to Mr. McDonnell in connection with his hire, as described in further detail below, as well as compensation for Mr. Capello due to his partial year employment with Biogen in 2020. | | Biotechnology Peers | |
100% of our NEOs’ 2020 annual long-term incentive (LTI) grants were performance-based and at-risk* Alexion Pharmaceuticals, Inc. Amgen Inc.
Celgene Corporation
Gilead Sciences Inc.
Vertex Pharmaceuticals International, Inc.
| | | 
| | • 60% earned based on achievement of three-year pipeline milestone performance goals • 40% earned based on achievement of three one-year financial goals relating to Non-GAAP adjusted free cash flow and revenue • Earned based on stock price performance over one-, two- and three-year periods |
| * | Does not include sign-on LTI awards granted to Mr. McDonnell in connection with his hire in August 2020. | Pharmaceutical Peers |
Our 2020 performance-based compensation payouts align with our commitment to strong performance and accountability. Our executive compensation program is structured to closely align with our business purpose and commitment to drive the creation of long-term stockholder value. Our C&MD Committee considered our achievements in 2020 as well as the challenges we faced and made adjustments to certain of the performance goals in our incentive compensation plans to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. At the same time, our C&MD Committee believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals and decreased the payouts under certain of our incentive plans for our NEOs. As a result, the payouts for our NEOs, as a percentage of target, for our 2020 annual bonus plan and the portions of our PSUs and our MSUs that were eligible to be earned based on 2020 performance were below target payout amounts, as described in further detail below. | | | | | | | | | 37 | | 
| |  |
| | AbbVie Inc.
Allergan plc
Bristol-Myers Squibb Company
Eli Lilly and Company
Merck & Co, Inc.
Mylan N.V.
Bausch Health Companies (f/k/a Valeant Pharmaceuticals Incorporated)
|
| 6 | | For each
Executive Compensation Matters (continued) | |
We believe that our 2020 executive compensation program, including the adjustments made by our C&MD Committee, demonstrates our commitment to linking compensation to Company performance and strategy during a challenging year while holding our executive officers accountable. 2020 Advisory Vote on Executive Compensation | | | At our 2020 annual meeting of stockholders, we continued to receive support for our executive compensation programs with approximately 83% of the companies invotes cast for approval of our peer group, where available, we analyze the company’s Compensation Discussion and Analysis and other data publicly filed during the prior year to identify the executives at such companies whose positions are comparable to those held by our executive officers. We then compile and analyze the data for each comparable position. Our competitive analysis includes the structure and design of the compensation programs as well as the targeted value of the compensation under these programs. For our executive officers other than our CEO, we may supplement the data from our peer group with published compensation surveys where appropriate. For 2018, consistent with past years, we used theWillisTowersWatson U.S. CDB Pharmaceutical and Health Sciences Executive Compensation Database survey (which we refer to as the Willis Towers Watson survey). We chose the Willis Towers Watson survey because of the number of companies in our peer group that participate in it, the number of positions reported by the survey that continue to be comparable to our executive positions and the high standards under which we understand the survey is conducted (including data collection and analysis methodologies). All of the companies in our peer group are represented in a special cross-section of the Willis Towers Watson survey focused on our peer group, other than Bausch Health Companies (formally known as Valeant Pharmaceuticals Incorporated), which did not participate in the survey.
annual Compensation Elements“say-on-pay” proposal. Our C&MD Committee determines the elements of compensation we provide to our executive officers. The elements of viewed this as positive support for our executive compensation programs and their objectives are as follows:alignment with long-term stockholder value creation and determined that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives. | | | | | | | | | | | Element | | | | Objective(s) | | | Base
Salary
| | • | | Provides a fixed level of compensation that is competitive with the external market and reflects each executive’s contributions, experience, responsibilities and potential to contribute to our future success.
| | | Annual
Bonus
Plan
| | • | | Aligns short-term compensation with the annual goals of the Company.
| | | | • | | Motivates and rewards the achievement of annual Company and individual performance goals that support short- and long-term value creation.
| | | Long-term Incentives
| | • | | Aligns executives’ interests with the long-term interests of our stockholders by linking the value of awards to increases in our stock price.
| | | | | • | | Motivates and rewards the achievement of stock price growth andpre-established corporate performance goals, including those with a longer-term focus.
| | | | | • | | Promotes executive retention and stock ownership and focuses executives on enhancing long-term stockholder value.
| | | Benefits
| | • | | Promotes health and wellness.
| | | | | • | | Provides financial protection in the event of disability or death.
| | | | | • | | Providestax-beneficial ways for executives to save towards their retirement and encourages savings through competitive matches to executives’ retirement savings.
| | |
Compensation Mix
Our C&MD Committee determines the general mix of the elements ofis committed to continually reviewing our executive compensation programs. It does not targetprograms on a specific mix of value for the compensation elements within these programs in either the program design or pay decisions. Rather, our C&MD Committee reviews the mix of compensation elementsproactive basis to ensure an appropriate level of performance-based compensation is apportioned to the short-term and even more to the long-term to ensure alignment with our business goals and performance. Additionally, our C&MD Committee believes the greater the leadership responsibilities, the greater the potential impact an individual will have on Biogen’s future strategic direction. Therefore, for our executive officers, including our NEOs, additional emphasis is placed on performance-based compensation, with a particular emphasis on LTI.
The 2018 compensation mix for Mr. Vounatsos and our other NEOs was highly performance-based andat-risk; 91% of 2018 compensation was performance-based for Mr. Vounatsos and 84% of 2018 compensation was
| | | | | 39 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
performance-based for our other NEOs, assuming target level achievement of applicable corporate performance goals and with LTI awards measured at target grant date
values, and excluding theone-time transition awards of RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie, as described in further detail below.
Performance Goals and Target Setting Process
Early each year, our C&MD Committee reviews and establishes the pay levels of each element of total compensation for our executive officers. Total compensation is comprised of base salary, annual bonus and LTI awards.
As part of this process, our C&MD Committee reviews the mix of compensation elements to ensure our performance-based compensation is apportioned appropriately and aligns with our business goals and performance. Our C&MD Committee also ensures that the performance metrics and goals are aligned with the annual business plan approved by our Board of Directors so there is fullongoing alignment of executive incentive goals with the goals that have been established for the year. Executive officers are also evaluated based on qualitative factors, such as individual, strategic and leadership achievements. The use of both quantitative and qualitative metrics, as well as the weighting of such metrics, effectively mitigates the impact of a single risk, such as dependence on drug pricing, pipeline performance or market share, on overall compensation.
| | | | | 40 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
A summary of the process our C&MD Committee follows in setting compensation is described below:
| | | | |  Target Setting Target Setting
| | 
 Monitoring & Tracking Monitoring & Tracking
• Our C&MD Committee closely monitors progress against the performance goals throughout the year and engages in dialogue with management on such progress.
| |  Results & Awards: Results & Awards:
C&MD Committee Actions
• Reviews and discusses the performance of our executive officers against their respective performance goals.
• Reviews and discusses the Company, team and individual performance of each executive officer, other than our CEO, as assessed by our CEO.
• Reviews and discusses our CEO’s recommended compensation levels for each executive officer, other than himself, in the context of such executive officer’s contributions to the Company and the other factors described above.
• Approves the final compensation for each executive officer other than our CEO, including base salary, annual bonus and LTI awards.
• Reviews CEO compensation and recommends to our Board of Directors for approval the compensation of our CEO, including base salary, annual bonus and LTI awards.
| • Our C&MD Committee and our CEO discuss potential goals for the upcoming year that are tied to the short- and longer-term strategic goals of the Company as well as individual goals for our executive officers.
• The annual business plan for the year is approved by our Board of Directors. As part of the approval process, our Board considers many factors relevant to our business, reputation and strategy, including pipeline and business development, pricing and patient access, market expectations and intellectual property risk.
• Our C&MD Committee ensures that the performance goals and targets under our compensation plans are aligned with the approved annual business plan.
• Payout levels for each performance goal are established by management and approved by our C&MD Committee.
• The performance goals are then applied to the compensation opportunities for our executive officers, including NEOs, so that there is full alignment of executive incentive goals with the goals that have been established for the year.
• Our C&MD Committee also reviews base salaries, bonus and LTI planning ranges, plan designs, benefits and peer group data.
|
| | | | | 41 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
2018 Base Salary
Our Board of Directors reviewed the base salaries of chief executive officers in our peer group and considered Mr. Vounatsos’ compensation mix, capabilities, performance and future expected contributions. Mr. Vounatsos’ base salary was set at $1,300,000, which positioned him below the market median when compared to the chief executive officers of our peer group.
Our C&MD Committee undertook a similar review when approving the base salaries for our other NEOs, which positioned them, on average, slightly below the market median compared to persons with comparable jobs within our peer group.
The annual base salary of each of our NEOs in 2018, compared to 2017, was as follows:
| | | | | | | | | | | | | | Name | | 2018 Salary | | | 2017 Salary | | | % Increase(1) | | | M. Vounatsos | | $ | 1,300,000 | | | $ | 1,100,000 | | | 18.2% | | | J. Capello(2) | | $ | 750,000 | | | $ | 750,000 | | | n/a | | | M. Ehlers | | $ | 834,094 | | | $ | 794,375 | | | 5.0% | | | S. Alexander | | $ | 749,177 | | | $ | 723,842 | | | 3.5% | | | P. McKenzie | | $ | 633,938 | | | $ | 603,750 | | | 5.0% | | |
(1) | Percentage increase reflects the annual merit increase and, in the case of Mr. Vounatsos, also includes a market adjustment.
|
(2) | Mr. Capello was hired in November 2017. The initial determination of his base salary took into account the Company’s peer group data.
|
2018 Performance-Based Plans and Goal Setting
Our executive compensation programs place a heavy emphasis on performance-based compensation.
We maintain a short-term incentive plan, known as our annual bonus plan, as well as an LTI plan.
Awards to our NEOs under our annual bonus plan have been made under our 2008 Performance-Based Management Incentive Plan, and awards under our LTI plan are granted under our 2017 Omnibus Equity Plan.
Awards made under our annual bonus plan are directly tied to the achievement of our corporate performance goals, which are aligned with the Company’s short- and long-term strategic plans, as well as individual performance goals.
Awards made under our LTI plan are directly tied to the performance of the price of our common stock, which aligns our executives’ long-term interests with the interests of our stockholders. A portion of our LTI awards are also tied to the Company’s financial performance, as described below under “Long-Term Incentives – 2018 PSUs.”
| |  | |
In setting our annual goals under our short- and long-term incentive plans, in addition to our internal forecasts, we consider analysts’ projections for our performance and the performance of companies in our peer group, as well as broad economic and industry trends. We strive to establish challenging targets that result in payouts at or above target levels only when Company performance warrants it. Our C&MD Committee is responsible for reviewing and approving our annual goals, targets and levels of payout (e.g., threshold, target and maximum) for our executive incentive compensation plans and for reviewing and determining actual performance results at the end of the applicable performance period.In setting and approving the corporate performance goals for our executive officers and for the Company under both the short- and long-term incentive plans,2020 our C&MD Committee also considers the alignment of such goals to our business plan, the degree of difficulty of attainment and the potential for the goals to encourage inappropriate risk-taking. Our C&MD Committee has determined that the structures ofreviewed our executive compensation programs do not putin light of market data, the results from our patients, investors or“say-on-pay” proposal at last year’s annual meeting of stockholders and the Company at any material risk.
Annual Bonus Plan
Our annual bonus plan is a cash incentive plan that rewards near-term financial, strategic and operationalCompany’s performance. Our C&MD Committee reviews the annual target bonus opportunities for eachwas satisfied that our existing executive officer by position each year to ensure such opportunities remain competitive.
Nocompensation programs further our pay-for-performance philosophy and, accordingly, did not recommend any significant changes were made in 2018 to the target annual bonus opportunities, as a percentage ofyear-end annual base salary, for any of our NEOs other than Mr. Vounatsos, whose target annual bonus opportunity was market adjusted and increased from 125% of base salary in 2017 to 140% of base salary in 2018. In accordance with our policy, target annual bonus opportunities for all of our other NEOs in 2018 were determined based on their positions as Executive Vice Presidents.
| | | | | 42 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
The target annual bonus opportunity as a percentage ofyear-end annual base salary each of our NEOs in 2018 compared to 2017 was as follows:
| | | | | | | | | | Name | | 2018 Target | | | 2017 Target | | M. Vounatsos | | | 140% | | | | 125% | | J. Capello | | | 70% | | | | 70% | | M. Ehlers | | | 70% | | | | 70% | | S. Alexander | | | 70% | | | | 70% | | P. McKenzie | | | 70% | | | | 70% | |
2018 Annual Bonus Plan Design
Awards for our NEOs under our 2018 annual bonus plan were based on the achievement of Company performance goals and individual performance goals.
At the beginning of 2018, our C&MD Committee set multiple Company performance goals for our 2018 annual bonus plan and provided for a payout multiplier, which we refer to as the Company Multiplier, ranging from 0% to 150%, for each Company goal based on the determination of the level of achievement of each goal and application of the weighting assigned to each goal, which determined the Company Multiplier applied to the bonus calculation.
The Company Multiplier ranged from 0% to 150% as follows:
| | | | | | | | | | | | | | Performance Multipliers | | Below Threshold | | Threshold | | Target | | Max | Company | | 0% | | 50% | | 100% | | 150% |
In addition, our 2018 annual bonus plan payouts were also based on an assessment of each NEO’s individual performance, taking into account his or her achievement of individual performance goals. Evaluating individual performance allows our C&MD Committee the discretion to increase or decrease each NEO’s bonus amount based on the NEO’s performance by applying an individual performance multiplier, ranging from 0% to 150%, which we refer to as the Individual Multiplier.
We determined the individual annual bonus payments for 2018 using the following calculation:
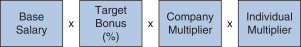
Our 2018 annual bonus plan provided that if the Company Multiplier was less than 50%, there would be no payout, regardless of individual performance, further strengthening ourpay-for-performance philosophy. Further, because the
Individual Multiplier and the Company Multiplier each have a maximum of 150%, the combined multiplier result for each NEO could not exceed 225%.
2018 Company Performance Goals and Results
Company performance goals were established at the start of 2018 with assigned weightings that reflected the Company’s focus on attaining both financial and strategic goals (pipeline performance, MS leadership, continued SMA launch excellence and enhancing our strategic alliances).
The goals and weightings we selected reflect the importance of linking reward opportunities to both near-term results and our progress in achieving longer-term goals.
The strategic goals we selected in 2018 were designed to measure the achievement of our annual strategic priorities relating to our commercial opportunities and pipeline progress. Our financial performance goals were based on the Company’s annual operating plan and long-range plan approved by our Board of Directors and with reference to analyst consensusexecutive compensation programs for Biogen revenues andNon-GAAP diluted EPS based on the most current analyst reports at the time we set our targets.
The following table presents our financial targets relative to analysts’ consensus for 2018:
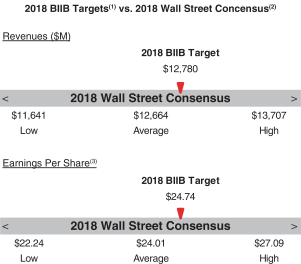
(1) Please see “2018 Annual Bonus Plan Company Performance Targets and Results Table” below for more details.
(2) Wall Street figures reflect estimates made in January 2018 for the Biogen fiscal year ending December 31, 2018.
(3) ReflectsNon-GAAP diluted EPS.
| | | | | 43 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
2018 Annual Bonus Plan Company Performance Targets and Results Table
Set forth below is a summary of the Company performance goals and weightings that our C&MD Committee established for our 2018 annual bonus plan and the degree to which we attained these Company performance goals. As described below, the Company Multiplier for the 2018 Annual Bonus Plan was 131%, reflecting the strong performance relative to ourpre-established goals.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Performance Range | | | | | | | | | | | | | | | Company Goals | | Weight | | | Threshold | | | Target | | | Max | | | Results | | | Company Multiplier | | FINANCIAL PERFORMANCE | | | | | | | | | | | | | | | | | | | | | | | | | Revenues | | | 20 | % | | $ | 12,310M | | | $ | 12,780M | | | $ | 13,250M | | | $ | 13,363M | (1) | | | 150.0 | % | Non-GAAP diluted EPS | | | 20 | % | | $ | 23.47 | | | $ | 24.74 | | | $ | 26.01 | | | $ | 26.89 | (1) | | | 150.0 | % | MARKET PERFORMANCE | | | | | | | | | | | | | | | | | Achieve Global MS Market Share | | | 15 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Below
Goal(2) |
| | | 91.8 | % | MS Leader in Customer Trust and Value Survey | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 125.0 | % | Achieve Global SMA Market Share | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 134.9 | % | PIPELINE DEVELOPMENT | | | | | | | | | | | | | | | | | Build and Advance Total Pipeline | | | 10 | % | |
| Specific pipeline goals
are not disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | | 110.0 | % | Achieve Aducanumab Phase 3 Enrollment | | | 5 | % | |
| Specific enrollment goals
are not disclosed for competitive reasons |
| |
| Above
Goal(4) |
| | | 105.0 | % | COLLABORATION | | | | | | | | | | | | | | | | | Improve and Expand Key Strategic Alliances | | | 10 | % | |
| Specific strategic
alliance goals are not
disclosed for competitive reasons |
| |
| Above
Goal(5) |
| | | 150.0 | % | Company Multiplier | | | | 131.0 | %* |
* | Numbers may not recalculate due to rounding.
|
Notes to 2018 Annual Bonus Plan Company Performance Targets and Results Table
(1) | These financial measures were based on our publicly reported revenues of $13,453 million and our publicly announcedNon-GAAP diluted EPS of $26.20, as adjusted as follows: for purposes of our 2018 annual bonus plan, revenues andNon-GAAP diluted EPS were adjusted to neutralize the effects of foreign exchange rate fluctuations.Non-GAAP diluted EPS was further adjusted to add back $1.21 to reflect the impact of additional research and development expense recognized in 2018 resulting from the 2018 Ionis Agreement and $0.07 to neutralize the unfavorable impact of the worldwide withdrawal of ZINBRYTA, partially offset by the subtraction of $0.59 related to higher than originally contemplated stock repurchases in 2018, as these charges were not originally contemplated at the time the Company performance goals were determined.
2020. |
(2) | Achievement of market goals for MS was below goal and achievement of MS leader and market goals for SMA were above goals. Specific details are not disclosed for competitive reasons.
|
Roles and Responsibilities (3) | The Company continued to expand andre-shapeRole of our C&MD Committee its pipeline ofpre-clinical and clinical stage programs through the advancement of internal programs, external business development activities and exceeding expectations with respect to the level of confidence in and momentum of its clinical stage portfolio. Specific details are not disclosed for competitive reasons.
|
(4) | Aducanumab Phase 3 clinical trial patient enrollment was above goal. Specific details are not disclosed for competitive reasons.
|
Our C&MD Committee, which is composed of three independent directors, oversees and administers our executive compensation programs. In making executive compensation decisions, our C&MD Committee reviews a variety of factors and data, most importantly our performance and individual executive performance, and considers the totality of compensation that may be paid or the value of which that may be granted. In addition, our C&MD Committee administers our annual bonus plan and our equity plans, reviews business achievements relevant to payouts under our compensation plans, makes recommendations to our Board of Directors with respect to compensation policies and practices as well as the compensation of our CEO and seeks to ensure that total compensation paid to our executive officers is fair, competitive and aligned with stockholder interests. Our C&MD Committee retains the right to hire outside advisors as needed to assist it in reviewing and revising our executive compensation programs. (5) | Key strategic alliance and acquisition activities were above goal. Specific details are not disclosed for competitive reasons.
|
The duties and responsibilities of our C&MD Committee are described on page 19 and can be found in our C&MD Committee’s written charter adopted by our Board of Directors, which can be found on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. | | | | | 44 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
2018 Individual Performance Goals and Results
The Individual Multiplier reflects each named executive officer’s overall individual performance rating as part of our performance assessment process. Unlike our formulaic calculation of corporate performance against Company performance goals in determining the Company Multiplier, each named executive officer’s Individual Multiplier is based on a subjective evaluation of his or her overall performance and consideration of the achievement of individual goals established at the beginning of the year. Goals may be both quantitative and qualitative. For 2018 Mr. Vounatsos recommended to our C&MD Committee an Individual Multiplier for each named executive officer other than himself based on his assessment of their individual contributions for the full year. Our C&MD Committee considered all of the information presented, discussed our CEO’s recommendations with him and its independent compensation consultant and applied its judgment to determine the Individual Multiplier for each named executive officer. Our Board of Directors determined Mr. Vounatsos’ Individual Multiplier based on its assessment of his performance.
In its evaluation, our C&MD Committee assigned Individual Multipliers to our named executive officers of between 115% and 140% based on the following accomplishments during 2018:
Michel Vounatsos
| | | Contributed to the achievement of record revenues of $13,453 million and $26.20Non-GAAP diluted EPS for the year ended December 31, 2018, versus targets of $12,780 million and $24.74, respectively.
Excelled in leading the Company in achieving its financial and business development goals.
Added substantial value to our business development activities and the diversification of our pipeline.
Contributed significantly to the demonstrated resilience in our MS business, the continued successful launch of SPINRAZA worldwide and the significant progress made in our biosimilars business.
Drove our ongoing improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values.
| 53,072 | | | | | — | | | | | 53,072 | | | | | * | | Michael R. McDonnell | | | | — | | | | | — | | | | | — | | | | | — | | Alfred W. Sandrock, Jr. | | | | 17,841 | | | | | — | | | | | 17,841 | | | | | * | | Susan H. Alexander | | | | 41,576 | | | | | — | | | | | 41,576 | | | | | * | | Chirfi Guindo | | | | 6,006 | | | | | — | | | | | 6,006 | | | | | * | | Jeffrey D. Capello(7) | | | | 3,118 | | | | | — | | | | | 3,118 | | | | | * | | Directors | | | | | | | | | | | | | | | | | | | | | Alexander J. Denner(8) | | | | 655,064 | | | | | 890 | | | | | 655,954 | | | | | * | | Caroline D. Dorsa | | | | 20,207 | | | | | 890 | | | | | 21,097 | | | | | * | | Maria C. Freire | | | | — | | | | | — | | | | | — | | | | | — | | William A. Hawkins | | | | 1,115 | | | | | 890 | | | | | 2,045 | | | | | * | | William D. Jones | | | | — | | | | | — | | | | | — | | | | | — | | Nancy L. Leaming | | | | 12,098 | | | | | 890 | | | | | 12,988 | | | | | * | | Jesus B. Mantas | | | | 2,053 | | | | | 890 | | | | | 2,943 | | | | | * | | Richard C. Mulligan | | | | 12,064 | | | | | 890 | | | | | 12,954 | | | | | * | | Robert W. Pangia(9) | | | | 19,742 | | | | | 890 | | | | | 20,632 | | | | | * | | Stelios Papadopoulos(10) | | | | 33,301 | | | | | 1,470 | | | | | 34,771 | | | | | * | | Brian S. Posner | | | | 6,870 | | | | | 890 | | | | | 7,760 | | | | | * | | Eric K. Rowinsky | | | | 16,179 | | | | | 890 | | | | | 17,069 | | | | | * | | Stephen A. Sherwin | | | | 15,438 | | | | | 890 | | | | | 16,328 | | | | | * | | Executive officers and directors as a group (21 persons)(11) | | | | 926,695 | | | | | 10,370 | | | | | 937,065 | | | | | * | |
| * | Represents beneficial ownership of less than 1% of our outstanding shares of common stock. |
| | | | | | | | | 26 | | 
| |  |
| | | | 4 | | Stock Ownership (continued) |
Contributed| (1) | The shares described as “owned” are shares of our common stock directly or indirectly owned by each listed person, rounded up to the achievementnearest whole share. |
| (2) | Includes RSUs that will vest within 60 days of record revenuesthe Ownership Date. |
| (3) | The calculation of $13,453 millionpercentages is based upon 150,554,556 shares outstanding on the Ownership Date, plus for each of the individuals listed above the shares subject to RSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and $26.20Non-GAAP diluted EPS for the year endedStock Units.” |
| (4) | Based solely on information as of December 31, 2018, versus targets2020, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 12, 2021, which also indicates that it has sole voting power over 15,443,182 shares and sole dispositive power over 15,822,066 shares. |
| (5) | Based solely on information as of $12,780 millionDecember 31, 2020, contained in a Schedule 13G/A filed with the SEC by BlackRock, Inc. on January 29, 2021, which also indicates that it has sole voting power with respect to 11,672,922 shares and $24.74, respectively. Significantly improvedsole dispositive power with respect to 13,419,601 shares.
|
| (6) | Based solely on information as of December 31, 2020, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 10, 2021, which also indicates that it has sole dispositive power with respect to 11,213,323 shares, shared voting power with respect to 259,934 shares and shared dispositive power with respect to 638,187 shares. |
| (7) | Mr. Capello ceased to be our Finance organization structureExecutive Vice President and key processes, including improved financial forecastingChief Financial Officer on August 15, 2020, and planningseparated from the Company on September 15, 2020. |
| (8) | Includes 643,000 shares beneficially owned by funds and taxaccounts managed by Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital). Dr. Denner is the Chief Investment Officer of Sarissa Capital and treasury planning. Added substantial valueultimately controls the funds and accounts managed by Sarissa Capital. By virtue of the foregoing, Dr. Denner may be deemed to our business development activities andindirectly beneficially own (as that term is defined in Rule 13d-3 of the diversificationExchange Act) the 643,000 shares that those entities beneficially own. Dr. Denner disclaims beneficial ownership of our pipeline.
Contributedthese shares except to the returnextent of approximately $4.4 billion to stockholders in 2018 through share repurchases under our 2018 Share Repurchase Program and 2016 Share Repurchase Program.
Contributed to excellent interactions with investors leading to transparent and trusted dialogue.
Contributed to improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values.
Supportedany pecuniary interest therein.
|
| (9) | Includes 16,000 shares beneficially owned by Robin Drive, LLC, of which Mr. Pangia’s wife is the Trustee. Mr. Pangia is retiring from our Board of Directors, the CEO and executive team. Michael Ehlers
Exceeded portfolio value and clinical development goals.
Significantly progressed and developed our pipeline.
Significantly improved our Research and Development organization structure, key processes and productivity.
Added new capabilities and talent to our Research and Development organization.
Excelled in leadership of our Research and Development organization.
Added substantial value to our business development activities.
Contributed to excellent interactions with investors leading to transparent and trusted dialogue.
Susan H. Alexander
Supported our Board of Directors, the CEO and executive team and SEC disclosure requirements.
Strengthened the intellectual property rights of our key assets, including our intellectual property related to TECFIDERA.
Excelled in leadership of our Legal and Compliance teams.
Contributed significantly on strategy and the resolution of general business issues affecting the Company, including our expansion into Asia Pacific and Latin America.
Supported the effective transitionas of the corporate services functions, including IT, to Mr. Capello.
Annual Meeting. |
| (10) | Paul F. McKenzieIncludes 28,206 shares held in limited liability companies of which Dr. Papadopoulos is the sole manager.
|
| (11) | Includes 688,175 shares held indirectly through trusts, funds, defined benefit plans or limited liability companies. |
Excelled in management of our large and complex manufacturing organization.
Maintained excellence in manufacturing plant quality.
| | | | | | | | | 27 | | | | | 45 | | 
| |  |
| | | | | | | Proposal 2 – Ratification of the Selection of Our Independent Registered Public Accounting Firm | | | | | | | |
Our Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the independent registered public accounting firm retained to audit our consolidated financial statements. Our Audit Committee has selected PwC as our independent registered public accounting firm for the fiscal year ending December 31, 2021. PwC has served as our independent registered public accounting firm since 2003. In order to assure continuing auditor independence, our Audit Committee periodically considers whether there should be a rotation of the independent registered public accounting firm. Further, in conjunction with the rotation of the auditing firm’s lead engagement partner required by applicable SEC rules, our Audit Committee and its Chair has in the past been, and in the future will be, directly involved in the selection of PwC’s new lead engagement partner. Our Audit Committee believes at this time that the continued retention of PwC to serve as our independent registered public accounting firm is in the best interest of Biogen and its stockholders. Although stockholder approval of our Audit Committee’s selection of PwC is not required, our Board of Directors believes that it is a matter of good corporate practice to solicit stockholder ratification of this selection. If our stockholders do not ratify the selection of PwC as our independent registered public accounting firm, our Audit Committee will reconsider its selection. Even if the selection is ratified, our Audit Committee always has the ability to change the engagement of PwC if it considers that a change is in Biogen’s best interest. Representatives of PwC will participate in the Annual Meeting, have the opportunity to make a statement if they so desire and be available to respond to appropriate questions. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE RATIFICATION OF THE SELECTION OF PRICEWATERHOUSECOOPERS LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR THE FISCAL YEAR ENDING DECEMBER 31, 2021. | | | | | | | | | 28 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit Committee Report The Audit Committee’s role is to act on behalf of our Board of Directors in the oversight of Biogen’s financial reporting, internal control and audit functions. The roles and responsibilities of the Audit Committee are set forth in the written charter adopted by our Board of Directors, which is posted on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. Management has primary responsibility for the financial statements and the reporting process, including the systems of internal control. In fulfilling its oversight responsibilities, the Audit Committee, among other things: reviewed and discussed with management the audited consolidated financial statements contained in Biogen’s 2020 Annual Report on Form 10-K; discussed with PwC, Biogen’s independent registered public accounting firm, the overall scope and plans for the audit; met with PwC, with and without management present, to discuss the results of its examination, management’s response to any significant findings, its observations of Biogen’s internal control, the overall quality of Biogen’s financial reporting, the selection, application and disclosure of critical accounting policies, new accounting developments and accounting-related disclosures, the key accounting judgments and assumptions made in preparing the financial statements and whether the financial statements would have materially changed had different judgments and assumptions been made and other pertinent items related to Biogen’s accounting, internal control and financial reporting; discussed with representatives of Biogen’s corporate internal audit staff, with and without management present, their purpose, authority, audit plan and reports; reviewed and discussed with PwC the matters required by the Public Company Accounting Oversight Board and the SEC; discussed with PwC its independence from management and Biogen, including the written disclosures and letter concerning independence received from PwC under applicable requirements of the Public Company Accounting Oversight Board. The Audit Committee has determined that the provision of non-audit services to Biogen by PwC is compatible with its independence; provided oversight and advice to management in connection with Biogen’s system of internal control over financial reporting in response to the requirements set forth in Section 404 of the Sarbanes-Oxley Act of 2002 and related regulations. In connection with this oversight, the Audit Committee reviewed a report by management on the effectiveness of Biogen’s internal control over financial reporting; and reviewed PwC’s Report of Independent Registered Public Accounting Firm included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, related to its audit of the effectiveness of internal control over financial reporting. In reliance on these reviews and discussions, the Audit Committee recommended to our Board of Directors that the audited consolidated financial statements be included in Biogen’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, for filing with the SEC. The Audit Committee of our Board of Directors: Caroline D. Dorsa (Chair) William A. Hawkins Nancy L. Leaming Stephen A. Sherwin, M.D. | | | | | | | | | 29 | | 
| |  |
| | | | 5 | | Audit Committee Matters (continued) |
Audit and Other Fees The following table shows fees for professional audit services billed to us by PwC for the audit of our annual consolidated financial statements for the years ended December 31, 2020 and December 31, 2019, and fees billed to us by PwC for other services provided during 2020 and 2019: | | | | | | | | | | | | Fees (amounts in thousands) | | 2020 | | | 2019 | | Audit fees | | $ | 5,882.0 | | | $ | 6,080.3 | | Audit-related fees | | | 48.2 | | | | 55.0 | | Tax fees* | | | 392.5 | | | | 641.8 | | All other fees | | | 9.9 | | | | 7.0 | | Total | | $ | 6,332.6 | | | $ | 6,784.1 | |
| * | Includes tax compliance fees of approximately $0.1 million in 2020 and 2019. |
Audit fees are fees for the audit of our 2020 and 2019 consolidated financial statements included in our Annual Reports on Form 10-K, reviews of our condensed consolidated financial statements included in our Quarterly Reports on Form 10-Q, review of the consolidated financial statements incorporated by reference into our outstanding registration statements and statutory audit fees in overseas jurisdictions. Audit-related fees are fees that principally relate to assurance and related services that are also performed by our independent registered public accounting firm. More specifically, these services include audits of employee benefit plan information, accounting consultations, due diligence and audits in connection with business development activity, internal control reviews and attest services related to financial reporting that are not required by statute or regulation. Tax fees are fees for tax compliance and planning services. All other fees in 2020 and 2019 also includelicense fees for a web-based accounting research tool. Policy on Pre-Approval of Audit and Non-Audit Services Our Audit Committee has the sole authority to approve the scope of the audit and any audit-related services as well as all audit fees and terms. Our Audit Committee must pre-approve any audit and non-audit services provided by our independent registered public accounting firm. Our Audit Committee will not approve the engagement of the independent registered public accounting firm to perform any services that the independent registered public accounting firm would be prohibited from providing under applicable securities laws, Nasdaq requirements or Public Company Accounting Oversight Board rules. In assessing whether to approve the use of our independent registered public accounting firm to provide permitted non-audit services, our Audit Committee tries to minimize relationships that could appear to impair the objectivity of our independent registered public accounting firm. Our Audit Committee will approve permitted non-audit services by our independent registered public accounting firm only when it will be more effective or economical to have such services provided by our independent registered public accounting firm than by another firm. Our Audit Committee annually reviews and pre-approves the audit, audit-related, tax and other permissible non-audit services that can be provided by the independent registered public accounting firm. After the annual review, any proposed services exceeding pre-set levels or amounts, or additional services not previously approved requires separate pre-approval by our Audit Committee or the Chair of our Audit Committee. Any pre-approval decision made by the Chair of our Audit Committee is reported to our Audit Committee at the next regularly scheduled Audit Committee meeting. Our Chief Financial Officer and our Chief Accounting Officer can approve up to an additional $50,000 in the aggregate per calendar year for categories of services that our Audit Committee (or the Chair through its delegated authority) has pre-approved. All of the services provided by PwC during 2020 and 2019 were pre-approved in accordance with this policy. | | | | | | | | | 30 | | 
| |  |
| | | | 6 | | Executive Compensation Matters |
| | | | | | | | | | | | Proposal 3 – Advisory Vote on Executive Compensation | | | | | | | |
Our Compensation Discussion and Analysis, which appears below, describes our executive compensation programs and the compensation decisions that our C&MD Committee and our Board of Directors made with respect to the 2020 compensation of our named executive officers. As required pursuant to Section 14A of the Exchange Act, our Board of Directors is asking that stockholders cast a non-binding, advisory vote FOR the following resolution: “RESOLVED, that the compensation paid to the Company’s named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.” Our Board of Directors is asking that our stockholders support this proposal. Although the vote you are being asked to cast is non-binding, we value the views of our stockholders, and our C&MD Committee and our Board of Directors will consider the outcome of the vote when making future compensation decisions for our named executive officers. As we describe in our Compensation Discussion and Analysis, our executive compensation programs embody a pay-for-performance philosophy that supports our business strategy and aligns the interests of our executives with those of our stockholders. In particular, our executive compensation programs reward financial, strategic and operational performance, and the goals set under our plans support our short- and long-range plans. In addition, to discourage excessive risk taking, we maintain policies for stock ownership, and our equity and annual bonus incentive plans have provisions providing for the recoupment of compensation. We also cap payments under our annual bonus plan, and we generally require multi-year vesting periods for long-term incentive awards. We will hold a non-binding, advisory vote of our stockholders on the compensation of our named executive officers every year until the next required stockholder vote on the frequency of such advisory vote. The next stockholder vote on the frequency of such advisory vote is expected to be held at the 2023 annual meeting of stockholders. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE APPROVAL OF THE RESOLUTION SET FORTH ABOVE. | | | | | | | | | 31 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Excelled in leadership of | COMPENSATION DISCUSSION AND ANALYSIS |
This Compensation Discussion and Analysis (CD&A) describes our compensation strategy, philosophy, policies and practices underlying our executive compensation programs for 2020. It also provides information regarding the compensation that was earned by and awarded to our 2020 named executive officers, whom we refer to collectively as “named executive officers” or “NEOs.” Our named executive officers include our current executive officers listed below as well as Jeffrey D. Capello*, our former Executive Vice President and Chief Financial Officer. | | | | | | | | | | | | |  | | | | Michel Vounatsos Chief Executive Officer | | | |  | | | | Susan H. Alexander Executive Vice President, Chief Legal Officer and Secretary | | | | | | | | | | | | | |  | | | | Michael R. McDonnell* Executive Vice President and Chief Financial Officer | | | |  | | | | Chirfi Guindo Executive Vice President, Global Product Strategy and Commercialization | | | | | | | | | | | | | |  | | | | Alfred W. Sandrock, Jr., M.D., Ph.D.** Executive Vice President, Research and Development | | | | | | | | |
| * | Mr. McDonnell was appointed as Executive Vice President and Chief Financial Officer effective August 15, 2020. Mr. Capello ceased to be our Pharmaceutical OperationsExecutive Vice President and Technology organization. Contributed significantlyChief Financial Officer on strategyAugust 15, 2020, and the resolution of general business issues affecting the Company.
Contributed to the significant progress in our biosimilars business.
Exhibited outstanding leadership, fostering a culture of continuous improvement and cost-consciousness.
In addition, our C&MD Committee reviews on a qualitative basis each named executive officer’s other contributions toseparated from the Company on September 15, 2020.
|
| ** | Dr. Sandrock was appointed as Executive Vice President, Research and Development on October 1, 2019. Prior to this appointment, Dr. Sandrock served as our business, leadership competenciesExecutive Vice President, Chief Medical Officer, and relative performance among our named executive officers.continued in this role, in addition to his duties as Executive Vice President, Research and Development, until January 27, 2020. |
We had a productive and successful 2020 as we continued to execute well against our corporate strategy. Our full year revenue for 2020 was $13.4 billion, a 6% decrease from the prior year primarily due to the entry of multiple TECFIDERA generic entrants in the U.S. with deeply discounted prices compared to TECFIDERA. The generic competition for TECFIDERA significantly reduced our TECFIDERA revenues during the year ended December 31, 2020, and is expected to have a substantial negative impact on our TECFIDERA revenues for as long as there is generic competition. Excluding TECFIDERA in the U.S., our global MS revenue, including OCREVUS royalties, remained relatively stable for 2020, as compared to 2019, demonstrating the resilience of our MS business in a competitive market. We continued our launch of VUMERITY in the U.S., which was the number two MS product and the number one oral in terms of new prescriptions in the U.S. as of December 31, 2020. We maintained our leadership in our SMA business despite increased competition and delays in SPINRAZA doses due, directly or indirectly, to the COVID-19 pandemic. Although our full year 2020 SPINRAZA revenue decreased 2% as compared to 2019, we continued to see growth outside of the U.S., with full year 2020 revenue outside the U.S. growing 9% as compared to 2019, and we believe that SPINRAZA will remain a foundation of care in the treatment of SMA. | | | | | | | | | 2018 Annual Bonus Plan Awards32
| | Our C&MD Committee determined that the final bonus awards under our 2018 annual bonus plan were as follows:
| |  |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Year-end Salary (A) x | | | Target Bonus% (B) x | | | Company Multiplier (C) x | | | Individual Multiplier (D) = | | | Bonus Award (E) | | | | | | | | | | | | | | M. Vounatsos | | $ | 1,300,000 | | | | 140 | % | | | 131 | % | | | 140 | % | | $ | 3,337,880 | | | | | | | | | | | | | | J. Capello | | $ | 750,000 | | | | 70 | % | | | 131 | % | | | 115 | % | | $ | 790,913 | | | | | | | | | | | | | | M. Ehlers | | $ | 834,094 | | | | 70 | % | | | 131 | % | | | 120 | % | | $ | 917,837 | | | | | | | | | | | | | | S. Alexander | | $ | 749,177 | | | | 70 | % | | | 131 | % | | | 125 | % | | $ | 858,744 | | | | | | | | | | | | | | P. McKenzie | | $ | 633,938 | | | | 70 | % | | | 131 | % | | | 135 | % | | $ | 784,784 | | | | | | | |
| | | | 6 | | Long-Term IncentivesExecutive Compensation Matters (continued)
|
Our full year 2020 biosimilars revenue increased 8% as compared to 2019. Although our biosimilars business was negatively impacted by pricing pressure and a slowdown in new treatments and reduced clinic capacity due to the COVID-19 pandemic, we were the leading anti-TNF biosimilar provider in Europe in 2020, and BENEPALI was the #1 prescribed etanercept product across Europe. We also made significant progress toward building a multi-franchise portfolio, with 10 programs now in either Phase 3 or filed across a number of key therapeutic areas, including regulatory filings for aducanumab in the U.S., the E.U. and Japan. We added or advanced 12 clinical programs in Alzheimer’s disease, MS, ALS, Parkinson’s disease and other movement disorders, depression and biosimilars and had a strong year for business development, including multiple new strategic collaborations. We provided value to our stockholders through the return of approximately $6.7 billion in capital through share repurchases, and we continued our leading efforts in environmental, sustainability and diversity issues. To help ensure the health and safety of our employees, we took several actions in response to the ongoing COVID-19 pandemic. In the U.S. and in most other key markets, our office-based employees began working from home in early March 2020, while we ensured essential staffing levels in our operations remained in place, including maintaining key personnel in our laboratories and manufacturing facilities. To provide a safe work environment for our employees, we have, among other things, increased our cleaning and sanitation routines on our campuses, implemented various social distancing measures on our campuses, created electronic health attestation forms, issued travel advisories to our employees consistent with government regulations and restricted participation of our employees in any events that have large gatherings. We have also suspended the vast majority of our in-person interactions by our customer-facing professionals in healthcare settings and are engaging with these customers remotely as we seek to continue to support healthcare professionals and patient care. Our C&MD Committee considered all of these achievements, and challenges, as they navigated compensation decisions not just for our executive officers but for all of our employees. As described below, our C&MD Committee exercised its discretion and made adjustments to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. Our C&MD Committee believed these adjustments were appropriate because the items were beyond the control of management, were not contemplated and/or could not be quantified due to uncertainty regarding magnitude and timing when the Company performance goals were originally set. Our C&MD Committee also believed that these adjustments were necessary to appropriately motivate and reward employees for their performance during a challenging year in which we continued to perform well despite the challenges that we faced. However, notwithstanding the attainment of our performance goals and the strength of management’s performance, our C&MD Committee also believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals. As a result, our C&MD Committee exercised its discretion and decreased the payouts under certain of our incentive compensation plans for the members of our Executive Committee, including all of our NEOs, as described below. Our C&MD Committee believes that our executive compensation program for 2020 is consistent with our compensation philosophies and principles described below and demonstrates our commitment to linking compensation to Company performance and strategy during a challenging year. | | | | | | | | | 33 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
2020 Highlights A brief summary of our 2020 business, financial and executive compensation highlights is as follows: Financial Performance The following chart provides a summary of our financial performance for 2020 compared to 2019: 
A reconciliation of our GAAP to Non-GAAP financial measures is provided in Appendix A to this Proxy Statement. Product and Pipeline Developments The following provides a summary of our product and pipeline developments for 2020: Applications for Marketing and Agency Actions Aducanumab In July 2020 we completed the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the approval of aducanumab. In August 2020 the FDA accepted the BLA and granted Priority Review with a Prescription Drug User Fee Act (PDUFA) action date on March 7, 2021. In January 2021 the FDA extended the review period for the BLA for aducanumab by three months. The updated PDUFA action date is June 7, 2021. In October 2020 the European Medicines Agency (EMA) accepted for review the Marketing Authorization Application (MAA) for aducanumab. In December 2020 the Ministry of Health, Labor and Welfare accepted for review the Japanese New Drug Application for aducanumab. SB11 (referencing LUCENTIS) In October 2020 the EMA accepted for review the MAA for SB11, a proposed ranibizumab biosimilar referencing LUCENTIS, and in November 2020 the FDA accepted the BLA for SB11. Ranibizumab is an anti-VEGF (vascular endothelial growth factor) for retinal vascular disorders, which are a leading cause of blindness. MS In March 2020 we made a regulatory submission to the EMA for a subcutaneous (SC) formulation of TYSABRI (natalizumab). In June 2020 we submitted a Supplemental Biologics License Application for a SC formulation of natalizumab to the FDA. In October 2020 the first patient in the Phase 1 study of BIIB107 (anti-VLA4) in MS was dosed. In November 2020 we submitted a MAA for VUMERITY (diroximel fumarate; DRF) to the EMA. | | | | | | | | | 34 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
In December 2020 the European Commission approved a new intramuscular injection route of administration for PLEGRIDY (peginterferon beta-1a) for the treatment of relapsing-remitting MS. Clinical Trials Alzheimer’s Disease and Dementia In March 2020 the first patient was dosed in the aducanumab re-dosing study, EMBARK, which is a global re-dosing clinical study designed to evaluate aducanumab in eligible Alzheimer’s disease patients who were actively enrolled in aducanumab studies (PRIME, EVOLVE, EMERGE and ENGAGE) in March 2019. In September 2020 the first patient was dosed in the Phase 3 AHEAD 3-45 clinical study of BAN2401 (lecanemab), an anti-amyloid beta antibody, in individuals with preclinical Alzheimer’s disease who have intermediate or elevated levels of amyloid in their brains. We are collaborating with Eisai on the development of BAN2401. Neuromuscular Disorders In March 2020 the first patient was dosed in the global DEVOTE study, which is evaluating the safety, tolerability and potential for even greater efficacy of SPINRAZA when administered at a higher dose than currently approved for the treatment of SMA. In September 2020 the first patient in a Phase 1 study of BIIB105 (ataxin-2 ASO), an antisense oligonucleotide (ASO) targeting ataxin-2 in ALS, was dosed. Movement Disorders In July 2020 the first patient in the Phase 1 study of BIIB101 (ION464), an ASO targeting alpha synuclein in multiple system atrophy, was dosed. Immunology In August 2020 the first patient was dosed in the Phase 3 program for dapirolizumab pegol (anti-CD40L) in patients with active systemic lupus erythematosus despite being treated by standard of care therapies. Dapirolizumab pegol is being developed in collaboration with UCB Pharma S.A. Biosimilars – Samsung Bioepis – Biogen’s Joint Venture with Samsung BioLogics In May 2020 Samsung Bioepis announced that the primary endpoints were met in the randomized, double-masked, Phase 3 trial comparing the efficacy, safety and immunogenicity of SB11 to the reference product (LUCENTIS). In June 2020 Samsung Bioepis initiated a Phase 3 study for SB15, a proposed aflibercept biosimilar referencing EYLEA. EYLEA is widely used to treat ophthalmologic conditions such as neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema (DME) and diabetic retinopathy in patients with DME. Discontinued Programs In March 2020 we announced that the Phase 2 OPUS study investigating natalizumab as an adjunctive therapy in adults with drug-resistant focal epilepsy did not meet its primary endpoint. Safety data were in-line with the known safety profile of natalizumab. Based on these results, we discontinued development of natalizumab in drug-resistant focal epilepsy. In October 2020 we announced that the Phase 2 AFFINITY study of opicinumab (anti-LINGO) in MS did not meet its primary or secondary endpoints. Based on these results, we discontinued development of opicinumab. Business Development In March 2020 we acquired BIIB118 (CK1 inhibitor), a novel CNS-penetrant small molecule inhibitor of casein kinase 1, for the potential treatment of patients with behavioral and neurological symptoms across various psychiatric and neurological diseases from Pfizer Inc. We are developing BIIB118 for the potential treatment of irregular sleep wake rhythm disorder in Parkinson’s disease and plan to develop BIIB118 for the potential treatment of sundowning in Alzheimer’s disease. | | | | | | | | | 35 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
In April 2020 we closed a collaboration and license agreement with Sangamo to develop and commercialize ST-501 for tauopathies, including Alzheimer’s disease; ST-502 for synucleinopathies, including Parkinson’s disease; a third neuromuscular disease target; and up to nine additional neurological disease targets to be identified and selected within a five-year period. The companies are leveraging Sangamo’s proprietary zinc finger protein technology delivered via adeno-associated virus to modulate the expression of key genes involved in neurological diseases. In October 2020 we closed a collaboration and license agreement with Denali to co-develop and co-commercialize Denali’s small molecule inhibitors of leucine-rich repeat kinase 2 (LRRK2) for Parkinson’s disease. In addition to the LRRK2 program, we also have an exclusive option to license two preclinical programs from Denali’s Transport Vehicle platform, including its Antibody Transport Vehicle (ATV): ATV enabled anti-amyloid beta program and a second program utilizing its Transport Vehicle technology. Further, we have a right of first negotiation on two additional Transport Vehicle-enabled therapeutics, should Denali decide to seek a collaboration for such programs. In December 2020 we closed a global collaboration and license agreement with Sage to jointly develop and commercialize BIIB125 (zuranolone) for the potential treatment of major depressive disorder and postpartum depression and BIIB124 (SAGE-324) for the potential treatment of essential tremor with potential in other neurological conditions such as epilepsy. Share Repurchase Activity In October 2020 our Board of Directors authorized a program to repurchase up to $5.0 billion of our common stock (2020 Share Repurchase Program). Our 2020 Share Repurchase Program does not have an expiration date. All share repurchases under our 2020 Share Repurchase Program will be retired. We returned approximately $6.7 billion to stockholders in 2020 through share repurchases under our 2020 Share Repurchase Program, our March 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in March 2019 to repurchase up to $5.0 billion of our common stock that was completed as of March 31, 2020, and our December 2019 Share Repurchase Program, which was a program authorized by our Board of Directors in December 2019 to repurchase up to $5.0 billion of our common stock that was completed as of September 30, 2020. Other Notable Achievements in the Workplace and Community | | | | | | | | | | | Terms | | Performance Stock Units (PSUs) | | Market Share Units (MSUs) | | | | Proportion of Annual Target Value | | 50% | | 50% | | | | | Settlement | | 60% stock settled | | 40% cash settled | | 100% stock settled | | | | | | Performance Period(s) | | 3 years (2018-2020) | | 1 year (each of 2018, 2019, 2020) | | 1 year, 2 years, 3 years (from grant date) | | | | | Metrics and Weighting | | AdjustedNon-GAAP diluted EPS: 30% Pipeline Milestone Performance: 30% | | Adjusted Free Cash Flow: 28% Revenues: 12% | | Stock Price: 100% | | | | | Threshold / Maximum Payout (% of Target Award) | | 50% / 200% | | 50% / 200% | | 50% / 200% | | | | | | Vesting | | 3-year Cliff Vesting | | 3-year Cliff Vesting | | Annual Ratable Vesting over 3 years (1/3 per year) | | | | |
| • | | All annual LTI awards grantedIn September 2020 we announced Healthy Climate, Healthy Lives, a $250.0 million, 20-year initiative to eliminate fossil fuels across our executives are performance-basedoperations and designed to reward long-term Company performance.
Our executive annual LTI program for 2018 consisted of PSUs and MSUs,collaborate with renowned institutions with the annual LTI total target grant value of awards being split evenly between PSUs and MSUs. The PSUs we awardedaim to executive officersimprove health, especially for the world’s most vulnerable populations. We are performance-based RSUs that are settled, as applicable, in cash and shares ofthe first Fortune 500 company to commit to become fossil fuel free across our common stock. The MSUs we awarded to executive officers are performance-based RSUs that are settled in shares of our common stock. The performance conditions applicable to these PSUs and MSUs are described in further detail below.operations by 2040.
|
The approximately €2.4 billion of healthcare savings in 2020 across Europe that we estimate was contributed by our three anti-TNF biosimilars. Named the number one biotechnology company on the Dow Jones Sustainability World Index for the fifth time. Launched our electric vehicle fleet program, expanding our battery electric vehicles to 12 and office chargers to 49 as of December 31, 2020. Recognized as a corporate sustainability leader with the Gold Class Sustainability Award from RobecoSAM. Used green chemistry processes and techniques to reduce our waste and energy consumption. Committed to a climate target consistent with reductions required to keep warming to 1.5°C and joined the Business Ambition to 1.5°C. Began engaging our employees and suppliers in the transition to a fossil fuel-free future with 100% renewable electricity targets for suppliers and sustainable benefit programs for employees. Earned a perfect score of 100% on the Human Rights Campaign’s Corporate Equality Index (a national benchmarking tool on corporate policies and practices pertinent to LGBTQ employees) for the seventh consecutive year and a perfect score of 100% on the Disability Equality Index for the third consecutive year. Continued our commitment to diversity, equity and inclusion. As of December 31, 2020, 48% of director-level positions and above were held by women, and, in the U.S., 28% were held by ethnic or racial minorities. Launched an enhanced strategy with the aim to boost diversity* in U.S. manager positions and above by 30% by the end of 2021. Engaged more than 57,000 students in hands-on learning to inspire their passion for science since the inception of Biogen’s Community Labs in 2002 with priority focus on underrepresented students. Our annual LTI target grant values are differentiated based on an executive’s individual performance, potential future contributions and market competitiveness, as well as other factors. In determining the annual LTI target grant value, our C&MD Committee reviews LTI awards of our peer group and also reviews the total compensation of our executive officers against our peer group. In general, we have a heavier weighting in executive compensation mix towards LTI awards. On average, annual LTI target grant values for our NEOs position their total compensation at or around the median values of our peer group in cases where there are comparable positions at the peer companies.
| | | | | | | | | 36 | | 
| |  |
| 46 | |  | |  |
| | | 5 | | 6 | | Executive Compensation Matters (continued) |
| * | We have an establishedPercent of U.S. manager positions and above held by Black, African American and Latinx employees as well as Asian employees where underrepresented.
|
2020 Executive Compensation Programs and Pay-for-Performance Alignment We believe our executive compensation programs are effectively designed and have worked well to implement a pay-for-performance culture that is aligned with the interests of our stockholders. In 2020 our executive compensation programs consisted of base salary, short- and long-term incentives and other benefits. 91% of our CEO’s and 85% of our other currently-employed NEOs’ (other than our CEO) 2020 target compensation was performance-based and at-risk. 
| * | Reflects annual salary, target bonus and target grant value of the 2020 annual long-term incentive awards. The NEO compensation mix excludes the one-time sign-on bonus paid to Mr. McDonnell in connection with his hire, as described in further detail below, as well as compensation for Mr. Capello due to his partial year employment with Biogen in 2020. |
100% of our NEOs’ 2020 annual long-term incentive (LTI) grants were performance-based and at-risk* | | | 
| | • 60% earned based on achievement of three-year pipeline milestone performance goals • 40% earned based on achievement of three one-year financial goals relating to Non-GAAP adjusted free cash flow and revenue • Earned based on stock price performance over one-, two- and three-year periods |
| * | Does not include sign-onLTI grant practice where LTI grants are made followingawards granted to Mr. McDonnell in connection with his hire in August 2020. |
Our 2020 performance-based compensation payouts align with our commitment to strong performance and accountability. Our executive compensation program is structured to closely align with our business purpose and commitment to drive the creation of long-term stockholder value. Our C&MD Committee considered our achievements in 2020 as well as the challenges we faced and made adjustments to certain of the performance goals in our incentive compensation plans to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, when the performance goals were originally established. At the same time, our C&MD Committee believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance goals and decreased the payouts under certain of our incentive plans for our NEOs. As a result, the payouts for our NEOs, as a percentage of target, for our 2020 annual bonus plan and the portions of our PSUs and our MSUs that were eligible to be earned based on 2020 performance were below target payout amounts, as described in further detail below. | | | | | | | | | 37 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
We believe that our 2020 executive compensation program, including the adjustments made by our C&MD Committee, demonstrates our commitment to linking compensation to Company performance and strategy during a challenging year while holding our executive officers accountable. 2020 Advisory Vote on Executive Compensation | | | At our 2020 annual meeting of stockholders, we continued to receive support for our executive compensation programs with approximately 83% of the completionvotes cast for approval of our annual “say-on-pay” proposal. Our C&MD Committee viewed this as positive support for our executive compensation programs and their alignment with long-term stockholder value creation and determined that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives. Our C&MD Committee is committed to continually reviewing our executive compensation programs on a proactive basis to ensure the ongoing alignment of such programs with the interests of our stockholders. | |  | |
| In 2020 our C&MD Committee reviewed our executive compensation programs in light of market data, the results from our “say-on-pay” proposal at last year’s annual meeting of stockholders and the Company’s performance. Our C&MD Committee was satisfied that our existing executive compensation programs further our pay-for-performance philosophy and, accordingly, did not recommend any significant changes to our executive compensation programs for 2020. |
Roles and Responsibilities Role of our C&MD Committee Our C&MD Committee, which is composed of three independent directors, oversees and administers our executive compensation programs. In making executive compensation decisions, our C&MD Committee reviews a variety of factors and data, most importantly our performance and individual executive performance, and considers the totality of compensation that may be paid or the value of which that may be granted. In addition, our C&MD Committee administers our annual bonus plan and our equity plans, reviews business achievements relevant to payouts under our compensation plans, makes recommendations to our Board of Directors with respect to compensation policies and practices as well as the compensation of our CEO and seeks to ensure that total compensation paid to our executive officers is fair, competitive and aligned with stockholder interests. Our C&MD Committee retains the right to hire outside advisors as needed to assist it in reviewing and revising our executive compensation programs. The duties and responsibilities of our C&MD Committee are described on page 19 and can be found in our C&MD Committee’s written charter adopted by our Board of Directors, which can be found on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. Role of the Independent Compensation Consultant Our C&MD Committee believes that independent advice is important in developing and overseeing our executive compensation programs. Pearl Meyer is currently engaged as our C&MD Committee’s independent compensation consultant. Pearl Meyer does not provide any other services to Biogen and engages in other matters as needed and as directed solely by our C&MD Committee. Reporting directly to our C&MD Committee, Pearl Meyer provides guidance on trends in CEO, executive and non-employee director compensation, the development of specific executive compensation programs and the composition of the Company’s compensation peer group used for market comparisons. Additionally, Pearl Meyer prepares a comprehensive report on CEO pay that compares each element of compensation to that of CEOs at companies in our peer group. Using this and other similar information, our C&MD Committee recommends, and our Board of Directors approves, the elements and target levels of our CEO’s compensation and our C&MD Committee approves the elements and target levels of compensation for our other executive officers. During 2020 Pearl Meyer also provided guidance to our C&MD regarding the impact of the COVID-19 pandemic on executive compensation programs, including at peer group companies. Our C&MD Committee assesses Pearl Meyer’s independence annually and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2020 that Pearl Meyer’s work did not raise any conflicts of interest and that Pearl Meyer remains independent under applicable rules. | | | | | | | | | 38 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Role of our CEO Each year our CEO provides an assessment of the performance of each executive officer, other than himself, during the prior year and recommends to our C&MD Committee the compensation to be paid or awarded to each executive. Our CEO’s recommendations are based on numerous factors, including: Company, team and individual performance; potential for future contributions; leadership competencies, skills and experience; external market competitiveness; internal pay comparisons; and other factors deemed relevant. To understand the external market competitiveness of the compensation for our executive officers, our CEO and our C&MD Committee review a report analyzing publicly-available information and surveys prepared by our internal compensation group and reviewed by Pearl Meyer. The report compares the compensation of each executive officer, other than our CEO, to data available for comparable positions at companies in our peer group and, in certain circumstances, the broader market, by compensation element (please see “External Market Competitiveness and Peer Group” below for further details). Our C&MD Committee considers all of the information presented, discusses the recommendations with our CEO and with Pearl Meyer and applies its judgment to determine the elements of compensation and target compensation levels for each executive officer other than the CEO. Our CEO also provides a self-assessment of his achievements for the prior year. Our C&MD Committee reviews and considers this in analyzing the CEO’s performance, and in recommending the compensation of our CEO for approval by our Board of Directors. Our CEO does not participate in any deliberations regarding his own compensation. Executive Compensation Philosophy and Objectives Our executive compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation that is competitive with our peer group in order to attract and retain extraordinary leaders who can perform at high levels and succeed in a demanding business environment. We aim to achieve this by designing programs that are: | • | | Mission Focused and Business Driven. Our executive compensation programs support the relentless pursuit of |
| | delivering meaningful and innovative therapies to patients by providing our executives with incentives to achieve the near- and long-term objectives of our business. Substantially all of our incentive compensation programs for our executives are tied directly, and meaningfully, to Company performance. Our objective is to emphasize the importance of achieving short-term goals while building and sustaining a foundation for long-term success. |
| • | | Competitively Advantageous. We benchmark our executive compensation programs against a peer group of biotechnology and pharmaceutical companies that we believe are representative of the companies we primarily compete with for talent, that are similar to us in business scope and size, including revenue and market capitalization, business focus and geographic scope of operations. We also review broader market data, as further described below, to provide additional context for compensation decisions. Peer group and market practices are among the many factors we take into account in developing executive compensation programs that we believe are most effective, and which enable us to recruit, retain and motivate our leadership team to achieve their best for Biogen and our stockholders. |
| • | | Performance Differentiated. We believe strongly in pay-for-performance and endeavor to significantly differentiate rewards by delivering the highest rewards to our best performers that exceed our expectations and lesser rewards to those who meet or do not meet our performance reviewsexpectations. |
| • | | Ownership Aligned. At Biogen, we believe every employee contributes to the success of the Company and, as such, every employee has a vested interest in the Company’s success. To reinforce this alignment with our stockholders, we strongly encourage stock ownership through our equity-based compensation programs. For members of our executive team, including our NEOs, who set and lead the future strategic direction of our Company, we ensure that a significant portion of their total pay opportunities are equity-based to maintain alignment between the interests of our executive officers as well asand our stockholders. |
| • | | Flexible. We are committed to providing flexible benefits designed to allow our diverse global workforce to have reward opportunities that meet their varied needs so that they are inspired to perform their very best on behalf of patients and stockholders each day. |
| | | | | | | | | 39 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
External Market Competitiveness and Peer Group We consider market practices and trends when determining executive compensation levels and compensation program designs at Biogen. We do not target a specific market percentile or simply replicate the market practice. Instead, we review external market practices as a reference point to assist us in providing programs designed to attract, retain and inspire extraordinary talent. Our C&MD Committee also uses a peer group and other market data to provide context for its executive compensation decision-making. Each year Pearl Meyer reviews the external market data and evaluates the composition of our peer group for appropriateness. Our C&MD Committee reviews the information provided from internal sources as well as the information provided by Pearl Meyer to select our peer group based on comparable companies that approximate (1) our scope of business, including revenue and market capitalization, (2) our global geographical reach, (3) our research-based business with multiple marketed products and (4) a comparable pool of talent for which we compete. The peer group used for our 2020 compensation decisions consisted of the biotechnology and pharmaceutical companies listed below, as we compete with companies in both of these sectors for executive talent. | | | Biotechnology Peers | Alexion Pharmaceuticals, Inc. Amgen Inc. Gilead Sciences Inc. Regeneron Pharmaceuticals, Inc. Vertex Pharmaceuticals Incorporated | | | Pharmaceutical Peers | AbbVie Inc. Allergan plc Bristol-Myers Squibb Company Eli Lilly and Company Merck & Co, Inc. Mylan N.V. Bausch Health Companies |
For each of the companies in our peer group, when available, we analyze the company’s Compensation Discussion and Analysis and other data publicly filed during the prior year to identify the executives at such companies whose positions are comparable to those held by our executive officers. We then compile and analyze the data for each comparable position. Our competitive analysis includes the structure and design of the executive compensation programs as well as the targeted value of the compensation under these programs. For our executive officers other than our CEO, we may supplement the data from our peer group with published compensation surveys. For 2020, consistent with past years, we used the WillisTowersWatson Pharmaceutical and Health Sciences Executive Compensation survey (which we refer to as the Willis Towers Watson survey). We chose the Willis Towers Watson survey because of the number of companies in our peer group that participate in it, the number of positions reported by the survey that continue to be comparable to our executive positions and the high standards under which we understand the survey is conducted (including data collection and analysis methodologies). All of the companies in our peer group are represented in a special cross-section of the Willis Towers Watson survey focused on our peer group other than Allergan plc, Regeneron Pharmaceuticals, Inc. and Vertex Pharmaceuticals Incorporated, none of which participated in the survey. | | | | | | | | | 40 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Compensation Elements Our C&MD Committee determines the elements of compensation we provide to our executive officers. The elements of our executive compensation programs and their objectives are as follows: | | | | | | | | | | | | Element | | | | Objective(s) | | | Base Salary | | • | | Provides a fixed level of compensation that is competitive with the external market reviewand reflects each executive’s contributions, experience, skills, responsibilities and potential to contribute to our future success. | | | Annual Bonus Plan | | • | | Aligns short-term compensation with the annual goals of equity practicesthe Company. | | | | • | | Motivates and rewards the achievement of annual Company and individual performance goals that support short- and long-term value creation. | | | Long-term Incentives | | • | | Aligns executives’ interests with the long-term interests of our peer group,stockholders by linking the value of awards to increases in our stock price. | | | | | • | | Motivates and rewards the achievement of stock price growth and pre-established Company performance goals, including those with a longer-term focus. | | | | | • | | Promotes executive retention and stock ownership and focuses executives on enhancing long-term stockholder value. | | | Benefits | | • | | Promotes health and wellness. | | | | | • | | Provides financial protection in the data fromevent of disability or death. | | | | | • | | Provides tax-beneficial ways for executives to save towards their retirement and encourages savings through competitive employer matches to executives’ retirement savings. | | |
Compensation Mix Our C&MD Committee determines the general mix of the elements of our executive compensation programs. It does not target a specific mix of value for the compensation elements within these programs in either the program design or pay decisions. Rather, our C&MD Committee reviews the mix of compensation elements to ensure an appropriate level of performance-based compensation is apportioned to the short-term and even more to the long-term to ensure alignment with our business goals, performance and stockholder interests. Additionally, our C&MD Committee believes the greater the leadership responsibilities, the greater the potential impact an individual will have on Biogen’s future strategic direction. Therefore, for our executive officers, including our NEOs, a greater portion of their compensation is performance-based, with a particular emphasis on LTI. The 2020 compensation mix for Mr. Vounatsos and our other NEOs was highly performance-based and at-risk; 91% of 2020 compensation was performance-based for Mr. Vounatsos and 85% of 2020 compensation was performance-based for our other currently-employed NEOs (other than Mr. Vounatsos), assuming target level achievement of applicable Company and individual performance goals and with LTI awards measured at target grant date values, and excluding the one-time sign-on bonus paid to Mr. McDonnell in connection with his hire. Performance Goals and Target Setting Process Early each year, our C&MD Committee reviews and establishes the pay levels of each element of total compensation for our executive officers. Total compensation is comprised of base salary, annual bonus and LTI awards. As part of this process, our C&MD Committee reviews the mix of compensation elements to ensure our performance-based compensation is appropriately apportioned and aligns with our business goals and performance and the annual business plan approved by our Board of Directors. In addition, the total compensation opportunity and mix of compensation elements for our executive officers are evaluated based on qualitative factors, such as individual, strategic and leadership achievements. Our CM&D Committee is aware of the risks associated with incentive compensation in general and specific factors, such as drug pricing, in particular, that may contribute to the achievement of particular performance goals. Our CM&D Committee considers these risks carefully when determining compensation and believes that the use and weighting of multiple metrics and the use of quantitative and qualitative metrics can mitigate these risks and create appropriate incentives to focus on achievement of the Company’s overall performance goals. | | | | | | | | | 41 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
A summary of the process our C&MD Committee follows in setting compensation is described below: | | | | |  Target Setting Target Setting
| | 
 Monitoring & Tracking Monitoring & Tracking
• Our C&MD Committee closely monitors progress against the Willis Towers Watson survey described above. Since 2004 we have madeperformance goals throughout the year based on reports and analysis on progress towards milestones and other success measures, and engages in dialogue with management on the level of progress. | |  Results & Awards: Results & Awards:
C&MD Committee Actions
• Reviews and discusses the performance of our annual LTI grants in Februaryexecutive officers against their respective performance goals. • Reviews and discusses the Company, team and individual performance of each year followingexecutive officer, other than our annual earnings release.CEO, as assessed by our CEO. We generally grant time-based RSUs
• Reviews and discusses our CEO’s recommended compensation levels for each executive officer, other than himself, in lieuthe context of PSUs at the time ansuch executive is hired if employment commences after June 30th. These grants are generally granted on the first trading day of the month following the date of hire. From time to time, we also grant time-based RSUs to recognize extraordinaryofficer’s contributions to the Company orand the other factors described above. • Approves the final compensation for transition or retention purposes.each executive officer other than our CEO, including base salary, annual bonus and LTI awards. In 2018
• Reviews CEO compensation and recommends the compensation of our CEO, including base salary, annual bonus and LTI target grant valuesawards, to our Board of Directors for approval. | • Our C&MD Committee and our CEO discuss potential goals for the upcoming year that are tied to the short- and longer-term strategic goals of the Company as well as individual goals for our executive officers. • The annual business plan for the year is approved by our Board of Directors. As part of the approval process, our Board considers many factors relevant to our business, reputation and strategy, including pipeline and business development, pricing and patient access, market expectations and intellectual property risk. • Our C&MD Committee ensures that the performance goals and targets under our compensation plans are aligned with the approved annual business plan. • Payout levels for each performance goal are established by management and approved by our C&MD Committee. • The performance goals are then applied to the compensation opportunities for our executive officers, including NEOs, wereso that there is full alignment of executive incentive goals with the goals that have been established for the year. • Our C&MD Committee also reviews base salaries, bonus and LTI planning ranges, plan designs, benefits and peer group and other broader market data. |
| | | | | | | | | 42 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
2020 Hiring- and Transition-Related Compensation Decisions Arrangements with Mr. McDonnell We appointed Mr. McDonnell as our Executive Vice President and Chief Financial Officer, effective as of August 15, 2020. In determining the annual and long-term compensation for Mr. McDonnell, our C&MD Committee followed the same process as it did when setting compensation for our other NEOs as described in this CD&A and also took into consideration the value of compensation that Mr. McDonnell would have been eligible to earn had he remained employed by his prior employer. After considering the compensation opportunities that Mr. McDonnell would be required to forfeit in order to join us, and in order to incentivize him to do so, our C&MD Committee granted Mr. McDonnell a one-time cash sign-on bonus of $1.0 million. Our C&MD Committee also approved an annual base salary for Mr. McDonnell of $850,000 and a target bonus of 75% of his annual base salary under our annual bonus plan, in each case, prorated for 2020. Mr. McDonnell’s one-time cash sign-on bonus is subject to repayment to the Company in the event Mr. McDonnell voluntarily terminates his employment or his employment is terminated by us for cause (as defined in our 2017 Omnibus Equity Plan) or for misconduct or poor performance, as determined by us in good faith, as follows: 100% of his cash sign-on bonus is subject to repayment if such termination occurs within the first year of his employment, 70% of his cash sign-on bonus is subject to repayment if such termination occurs within the second year of his employment and 35% of his cash sign-on bonus is subject to repayment if such termination occurs within the third year of his employment, in each case, net of applicable tax withholdings. In connection with Mr. McDonnell’s appointment, our C&MD Committee also granted him a LTI award in September 2020, which consisted of RSUs and MSUs with an aggregate grant date target value of $4.5 million. The terms of the RSUs and MSUs awards granted to Mr. McDonnell are described below under the heading “Long-Term Incentives.” Arrangements with Mr. Capello Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer on August 15, 2020, and separated from the Company on September 15, 2020. We provided him the severance benefits required under our executive severance policy for Executive Vice Presidents, which con- sisted of a lump sum payment of $1,892,625 (16 months of base salary and target bonus) and continuation of certain subsidized medical, dental and vision benefits until the earlier of (1) December 31, 2021, or (2) the date on which he becomes eligible to receive benefits through another employer. In addition, in recognition of Mr. Capello’s contributions to the Company and to facilitate a successful transition to Mr. McDonnell, our CM&D Committee waived the requirement that Mr. Capello repay 35% of his cash sign-on bonus and approved an additional cash payment to him of $2.6 million. 2020 Base Salary For 2020 our Board of Directors reviewed the base salaries of chief executive officers in our peer group and considered Mr. Vounatsos’ compensation mix, capabilities, performance, future expected contributions and positioning relative to the peer group. Mr. Vounatsos’ annual base salary was increased to $1.5 million, which positioned him below the market median when compared to the chief executive officers of our peer group. Our C&MD Committee undertook a similar review when approving the annual base salaries for our other NEOs, which positioned them, on average, below the market median compared to persons with comparable jobs within our peer group. The annual base salary of each of our NEOs in 2020, compared to 2019, is as follows: | | | | | | | | | | | | | | Name | | 2020 Salary | | | 2019 Salary | | | % Increase(1) | | | M. Vounatsos | | $ | 1,500,000 | | | $ | 1,400,000 | | | 7.1% | | | M. McDonnell(2) | | $ | 850,000 | | | | n/a | | | n/a | | | A. Sandrock | | $ | 901,680 | | | $ | 803,136 | | | 12.3% | | | S. Alexander | | $ | 814,168 | | | $ | 775,398 | | | 5.0% | | | C. Guindo | | $ | 567,100 | | | $ | 530,000 | | | 7.0% | | | J. Capello | | $ | 811,125 | | | $ | 787,500 | | | 3.0% | | |
| (1) | Percentage increase reflects the annual merit increase and, in the case of Dr. Sandrock, also includes a market adjustment. |
| (2) | Mr. McDonnell was hired in 2020. The initial determination of his base salary took into account the Company’s peer group data. |
2020 Performance-Based Plans and Goal Setting Our executive compensation programs place a heavy emphasis on performance-based compensation. We maintain a short-term incentive plan, known as our annual bonus plan, as well as a LTI plan. | | | | | | | | | Name | | Annual LTI
Target
Grant Value
| | | | | | | | | M. Vounatsos
| | $11,500,000 | | | | | | | | | J. Capello(1)
| | n/a | | | | | | | | | M. Ehlers(2)
| | $ 3,750,000 | | | | | | | | | S. Alexander(2)
| | $ 3,200,000 | | | | | | | | | P. McKenzie(2)
| | $ 3,000,000 | | | | | 43 | | 
| |  |
| 6 | | Notes to the 2018 Annual LTI Awards Table
(1) | In lieu of a 2018 annual LTI award, Mr. Capello received a new hire grant in January 2018, which consisted of PSUs and MSUs with an aggregate grant date target value of $3.0 million. The initial determination of these awards took into account the Company’s peer group data.Executive Compensation Matters (continued)
|
(2) | In addition to the annual LTI award, Dr. Ehlers, Ms. Alexander and Dr. McKenzie each received aone-time transition award of RSUs, as described in further detail below.
Awards to our NEOs under our annual bonus plan were made under our 2020 Performance-Based Management Incentive Plan, and awards under our LTI plan were granted under our 2017 Omnibus Equity Plan. Awards made under our annual bonus plan are directly tied to the achievement of our Company performance goals, which are aligned with the Company’s short- and long-term strategic plans, as well as individual performance goals. Awards made under our LTI plan are directly tied to the performance of the price of our common stock, which aligns our executives’ long-term interests with the interests of our stockholders. A portion of our LTI awards is also tied to the Company’s financial performance, as described below under “Long-Term Incentives – 2020 PSUs.” In setting our annual goals under our short- and long-term incentive plans, in addition to our internal forecasts, we consider analysts’ projections for our performance and the performance of companies in our peer group as well as broad economic and industry trends. We strive to establish challenging targets that result in payouts at or above target levels only when Company performance warrants it. Our C&MD Committee is responsible for reviewing and approving our metrics, goals, targets and levels of payout (e.g., threshold, target and maximum) for our executive incentive compensation plans and awards and for reviewing and determining actual performance results at the end of the applicable performance period. In setting and approving the performance goals for our executive officers and for the Company under both the short- and long-term incentive plans, our C&MD Committee also considers the alignment of such goals to our business plan, the degree of difficulty of attainment and the potential for the goals to encourage inappropriate risk-taking. Our C&MD Committee has determined that the structures of our executive compensation programs do not put our patients, investors or the Company at any material risk. Annual Bonus Plan Our annual bonus plan is a cash incentive plan that rewards near-term financial, strategic and operational performance. Our C&MD Committee reviews the annual target bonus opportunities for each executive officer by position each year to ensure such opportunities remain competitive. No changes were made in 2020 to the target annual bonus opportunities, as a percentage of year-end annual base salary, for any of our NEOs. Mr. McDonnell’s target annual bonus opportunity was set in connection with his hire, based on the factors described above, and was prorated for 2020 based on the portion of 2020 he was employed by us. The target annual bonus opportunity as a percentage of year-end annual base salary for each of our NEOs in 2020 compared to 2019 was as follows: | | | | | | | | | | Name | | 2020 Target | | | 2019 Target | | M. Vounatsos | | | 150% | | | | 150% | | M. McDonnell | | | 75% | | | | n/a | | A. Sandrock | | | 75% | | | | 75% | | S. Alexander | | | 70% | | | | 70% | | C. Guindo | | | 70% | | | | 70% | | J. Capello(1) | | | 75% | | | | 75% | |
| (1) | Mr. Capello ceased to be employed by the Company during 2020 and, as a result, was ineligible for a payout under our 2020 annual bonus plan. |
2020 Annual Bonus Plan Design Awards for our NEOs under our 2020 annual bonus plan were based on the achievement of Company performance goals and individual performance goals. At the beginning of 2020 our C&MD Committee set multiple Company performance goals for our 2020 annual bonus plan and a payout multiplier, which we refer to as the Company Multiplier, ranging from 0% to 150%. The Company Multiplier was applied to each Company goal based on the determination of the level of achievement of each goal and application of the weighting assigned to each goal to determine the total Company Multiplier applied to the bonus calculation. The Company Multiplier ranged from 0% to 150% as follows: | | | | | | | | | | | | | | Performance Multipliers | | Below Threshold | | Threshold | | Target | | Max | Company | | 0% | | 50% | | 100% | | 150% |
In addition, our 2020 annual bonus plan payouts were based on an assessment of each NEO’s individual performance, taking into account his or her achievement of pre-determined individual performance goals. Evaluating individual performance allows our C&MD Committee the discretion to increase or decrease each NEO’s bonus amount based on the NEO’s performance by applying an individual performance multiplier, ranging from 0% to 150%, which we refer to as the Individual Multiplier. | | | | | | | | | 44 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
We determined the individual annual bonus payments for 2020 using the following calculation: 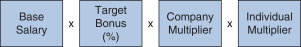
Our 2020 annual bonus plan provided that if the Company Multiplier was less than 50%, there would be no payout, regardless of individual performance, consistent with our pay-for-performance philosophy. Further, because the Individual Multiplier and the Company Multiplier each have a maximum of 150%, the combined multiplier result for each NEO could not exceed 225%. 2020 Company Performance Goals and Results Company performance goals were established in the early part of 2020 with assigned weightings that reflected the Company’s focus on attaining both financial and strategic goals (pipeline performance, MS leadership, accelerate neuromuscular leadership, prepare for Alzheimer’s disease leadership, biosimilars growth and executing strategic transactions that progress our multi-franchise neuroscience portfolio and/or optimize capital allocation). The goals and weightings selected reflect the importance of linking reward opportunities to both near-term results and our progress in achieving longer-term goals. The strategic performance goals selected in 2020 were designed to measure the achievement of our annual strategic priorities relating to our commercial opportunities and pipeline progress. Our financial performance goals were based on the Company’s annual operating plan and long-range plan approved by our Board of Directors and with reference to analyst consensus for Biogen revenue and Non-GAAP diluted earnings per share (EPS) based on the most current analyst reports at the time we set our targets. The following table presents our financial targets relative to analysts’ consensus for 2020: 
| (1) | Please see “2020 Annual Bonus Plan Company Performance Targets and Results Table” below for more details. |
| (2) | Wall Street figures reflect estimates made in January 2020 for the Biogen fiscal year ending December 31, 2020. |
| (3) | Reflects Non-GAAP diluted EPS. |
The actual value that will be realized from PSU awards depends on the degree of achievement of performance goals, with 60% of the PSUs (based on the grant date target value) settled in shares of our common stock based upon achievement of cumulative three-year financial and pipeline metrics and the remaining 40% of the PSUs settled in cash based upon the achievement of two annual financial metrics that are determined at the beginning of each relevant year. The actual value that will be realized from MSU awards depends on our30-day average common stock price growth between the grant date and each of the dates such awards vest. Our common stock price is influenced by the Company’s performance as well as external market factors.
2018 PSUs
PSUs comprised 50% of our executives’ target LTI for 2018. PSUs are performance-based RSUs that have three-year cliff vesting in furtherance of the Company’s long-termpay-for-performance philosophy and to encourage employee retention. PSUs align executive compensation to Company goals through performance against a combination of financial and pipeline milestone performance metrics. The actual value (if any) of PSUs will not be realized by the NEOs until the three-year period ends and then only if the applicable performance goals are achieved.
For our 2018 PSU awards, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock based on achievement of financial and pipeline performance goals over a three-year performance period (the 2018 Stock-Settled PSUs). The remaining 40% of the PSUs will be settled in cash based on the achievement of three sets ofone-year financial goals (the 2018 Cash-Settled PSUs) and continued employment through the vesting date. Our 2018 PSU awards are scheduled to vest in February 2021.
For our 2018 PSU awards, the number of PSUs earned at the end of the three-year performance period will be determined as follows:
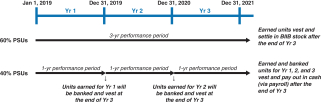
In designing our 2018 PSU LTI program, our C&MD Committee acknowledged the need to balance driving long-term performance and investing for the future with achieving key milestones along the way. Cash payments are primarily aligned with and reward more recent performance, while equity awards encourage our executives to continue to deliver results over a longer period of time and also serve as a retention tool. Accordingly, our C&MD Committee determined that moving compensation for our executive officers further away from cash and towards equity awards with longer-term goals would further align their interests with those of Biogen’s stockholders in creating long-term stockholder value.
2020 Annual Bonus Plan Company Performance Targets and Results Table Set forth below is a summary of the Company performance goals and weightings that our C&MD Committee established for our 2020 annual bonus plan and the degree to which we attained these Company performance goals. At the time our C&MD Committee established the Company performance goals in the beginning of the year, we did not know what impact, including the timing, that generic competition for TECFIDERA would have on our 2020 financial results as it was dependent on a number of factors beyond our control, including decisions in the patent infringement proceedings relating to TECFIDERA Orange-Book listed patents pursuant to the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, in West Virginia and Delaware. Due to this uncertainty, our C&MD Committee exercised its discretion to make certain adjustments to the results to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, at the time the Company performance goals were originally determined. In particular, the entry of multiple TECFIDERA generic entrants in the U.S. with deeply discounted prices compared to TECFIDERA had real and negative impacts on our financial results. | | | | | | | 47 | | 45 | | 
| |  |
| | | | 6 | | | | Executive Compensation Matters (continued) | 5 |
Our C&MD Committee believed these adjustments were appropriate because the items were beyond the control of management, were not contemplated and/or could not be quantified due to uncertainty regarding magnitude and timing when the Company performance goals were originally set. Our C&MD Committee also believed that adjustments were necessary to appropriately motivate and reward employees for their performance during a challenging year in which we continued to perform well despite the challenges that we faced. Based on our overall performance, and following these adjustments, the Company Multiplier for the 2020 Annual Bonus Plan was 106%. However, notwithstanding the attainment of these performance goals and the strength of management’s performance, our C&MD Committee believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance. As a result, our C&MD Committee exercised its discretion and decreased the Company Multiplier for the members of our Executive Committee, including all of our NEOs, by 11%, which resulted in a Company Multiplier for our NEOs of 95%. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Performance Range | | | | | | | | | | | | | | | Company Goals | | Weight | | | Threshold | | | Target | | | Max | | | Results | | | Company Multiplier | | FINANCIAL PERFORMANCE | | | | | | | | | | | | | | | | | | | | | | | | | Revenue | | | 20 | % | | $ | 13,280M | | | $ | 14,102M | | | $ | 14,923M | | | $ | 13,952M | (1) | | | 90.9 | % | Non-GAAP diluted EPS | | | 20 | % | | $ | 29.26 | | | $ | 32.37 | | | $ | 35.48 | | | $ | 33.30 | (1) | | | 114.9 | % | MARKET PERFORMANCE | | | | | | | | | | | | | | | | | Achieve Global MS Market Share | | | 7.5 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 116.5 | % | MS Leader in Customer Trust and Value Survey | | | 2.5 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | | 150.0 | % | Achieve Global SMA Market Share | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Goal Not
Met(3) |
| | | 0 | % | Prepare for Alzheimer’s Disease Leadership | | | 15 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | | 115.0 | % | Achieve anti-TNF Biosimilars Market Share | | | 2.5 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Below
Goal(3) |
| | | 96.0 | % | PIPELINE DEVELOPMENT | | | | | | | | | | | | | | | | | Build and Advance Total Pipeline | | | 15 | % | |
| Specific pipeline goals
are not disclosed for competitive reasons |
| |
| Above
Goal(4) |
| | | 140.0 | % | COLLABORATION | | | | | | | | | | | | | | | | | | | | | | Execute Strategic Transactions that Progress our Multi-Franchise Neuroscience Portfolio and/or Optimize Capital Allocation | | | 7.5 | % | |
| Specific strategic transactions
goals are not disclosed for competitive reasons |
| |
| Above
Goal(5) |
| | | 150.0 | % | Company Multiplier | | | | 106.0 | %* | Adjusted Company Multiplier for NEOs | | | | 95.0 | %*(6) |
| * | Numbers may not recalculate due to rounding. |
Notes to 2020 Annual Bonus Plan Company Performance Targets and Results Table | (1) | These financial measures were based on our publicly reported revenue of $13,445 million and our publicly announced Non-GAAP diluted EPS of $33.70, as adjusted as follows: for purposes of our 2020 annual bonus plan, revenue was adjusted to neutralize the effects of foreign exchange rate fluctuations and exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020. Non-GAAP diluted EPS was further adjusted to subtract $2.62 related to share repurchases in 2020 under our 2020 Share Repurchase Program, our December 2019 Share Repurchase Program and our March 2019 Share Repurchase Program and $0.28 related to the delay in the payment of a milestone payment related to the potential approval of aducanumab, partially offset |
| | | | | | | | | 46 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| by the addition of $2.50 to exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020. These items were either not originally contemplated or their magnitude or timing was uncertain at the time the Company performance goals were originally established. |
| (2) | 2018 PSU Awards TableOur market goal for MS was adjusted to exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020, which was not originally contemplated at the time the Company performance goals were determined. Specific details are not disclosed for competitive reasons.
|
| (3) | Set forthOur achievement of MS leader and preparation for Alzheimer’s disease leadership were above goals, achievement of market goals for biosimilars was below is a summarygoal and we did not meet our market goals for SMA. Specific details are not disclosed for competitive reasons.
|
| (4) | The Company continued to expand and re-shape its pipeline of pre-clinical and clinical stage programs through the advancement of internal programs, external business development activities and exceeding expectations with respect to the level of confidence in and momentum of its clinical stage portfolio. Specific details are not disclosed for competitive reasons. |
| (5) | We exceeded our key strategic alliance and business development goal. Specific details are not disclosed for competitive reasons. |
| (6) | Notwithstanding the attainment of these performance metricsgoals and weightings thatthe strength of management’s performance, our C&MD Committee establishedbelieved it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance compared to the original performance. As a result, our C&MD Committee exercised its discretion and decreased the Company Multiplier for the members of our Executive Committee, including all of our NEOs, by 11%, which resulted in a Company Multiplier for our 2018 PSU awards and the degree to which we achieved the performance goals for the 2018 trancheNEOs of the 2018 Cash-Settled PSUs. Based on the results outlined in the table below, the multiplier for the 2018 tranche of the 2018 Cash-Settled PSUs was 192%95%. |
2020 Individual Performance Goals and Results The Individual Multiplier reflects each named executive officer’s overall individual performance rating that is determined as part of our performance assessment process. Unlike our calculation of performance against Company performance goals in determining the Company Multiplier, each named executive officer’s Individual Multiplier, other than Mr. Vounatsos, is based on a subjective evaluation of his or her overall performance and consideration of the achievement of individual goals established at the beginning of the year. Goals may be both quantitative and qualitative. For 2020 Mr. Vounatsos recommended to our C&MD Committee an Individual Multiplier for each currently-employed NEO other than himself based on his assessment of their individual contributions for the full year. Our C&MD Committee considered all of the information presented, discussed our CEO’s recommendations with him and Pearl Meyer and applied its judgment to determine the Individual Multiplier for each currently-employed NEO. Our Board of Directors determined Mr. Vounatsos’ Individual Multiplier based on its assessment of his performance. In its evaluation, our C&MD Committee assigned Individual Multipliers to our named executive officers of between 112% and 140% based on the following accomplishments during 2020: Michel Vounatsos Excelled in leading the Company in achieving its financial and business development goals, executing on its corporate strategy and advancing Biogen’s values. Contributed to our business development activities and the diversification of our pipeline. Contributed to the demonstrated resilience in our MS business, the continued successful launch of SPINRAZA worldwide and the expansion of our biosimilars business. Supported the filing of applications to the FDA, the EMA and the Japanese Ministry of Health, Labor and Welfare for marketing approval of aducanumab as well as the preparation for the potential launch of aducanumab. Drove our ongoing improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values. Supported our continued leadership in corporate responsibility, including through the launch of Healthy Climate, Health Lives and diversity, equity and inclusion initiatives. Michael McDonnell Successfully transitioned into the role of our Chief Financial Officer. Significantly improved our Finance and IT organization structure, including improvements in our core processes to improve operating efficiencies. Added new capabilities and talent to our Finance organization. Contributed to our business development activities and the diversification of our pipeline. Contributed to interactions with investors that led to transparent and productive dialogue. Supported our Board of Directors, the CEO and executive team. | | | | | | | | | | | | | | | | | | | 47 | | 
| |  | | | | | | | | Percentage of
PSU Award | | Percentage of
|
PSU Target
Value / Total
LTI Target
Value
| | Performance Metrics | | Performance
Metrics
Weight | | | Performance
Period | | | Target
Performance
| | Actual
Performance
| | | | 6 | | Executive Compensation Matters (continued) | Stock-
Settled: 60%
| | 60% / 30% | | Adjusted Non-GAAP diluted EPS
Pipeline Milestone Performance
| |
| 30%
30% |
| |
| 2018-2020
2018-2020 |
| | Specific goals are not disclosed for competitive reasons | Cash-
Settled: 40%
| | 40% / 20% | | Adjusted Free Cash Flows
|
Alfred W. Sandrock, Jr. Supported the significant progression and development of our pipeline. Supported the filing of applications to the FDA, the EMA and the Japanese Ministry of Health, Labor and Welfare for marketing approval of aducanumab. Contributed to our business development activities and the diversification of our pipeline. Significantly improved our Research & Development organization structure, key processes and productivity. Excelled in leadership of our Research & Development organization. Contributed to interactions with investors that led to transparent and productive dialogue. Susan H. Alexander Supported our Board of Directors, the CEO and executive team. Led our compliance with SEC disclosure requirements. Provided strategic legal advice to support the filing of applications to the FDA, the EMA and the Japanese Ministry of Health, Labor and Welfare for marketing approval of aducanumab as well as the preparation for the potential launch of aducanumab. Co-led our response to the COVID-19 pandemic and established guidance for on-site safety of personnel. Excelled in leadership of our Legal and Compliance teams, fostering a culture of diversity, equity and inclusion and advancing Biogen’s values. Provided transaction and litigation support, including supporting key business development and regulatory matters. Chirfi Guindo Excelled in leadership of our Global Product Strategy and Commercialization organization and our Government Affairs, Value and Access and Corporate Affairs organizations. Contributed to the continued successful expansion of SPINRAZA worldwide and the continued launch of VUMERITY. Supported the preparation for the potential launch of aducanumab. Contributed to our strategy, product development and lifecycle management in a competitive environment. Supported our continued geographic expansion, including in emerging growth markets. Exhibited outstanding leadership, fostering a culture of diversity, equity and inclusion, including through the launch of Healthy Climate, Health Lives and diversity, equity and inclusion initiatives, as well as focusing on continuous improvement and implementing operational efficiencies. In addition, our C&MD Committee reviews on a qualitative basis each named executive officer’s other contributions to the Company and our business, leadership competencies and relative performance among all of our executive officers in determining Individual Multipliers. 2020 Annual Bonus Plan Awards Our C&MD Committee determined that the final bonus awards under our 2020 annual bonus plan were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Year-end Salary (A) x | | | Target Bonus% (B) x | | | Company Multiplier (C) x | | | Individual Multiplier (D) = | | | Bonus Award (E) | | | | | | | | M. Vounatsos | | $ | 1,500,000 | | | | 150 | % | | | 95 | % | | | 120 | % | | $ | 2,565,000 | | | | | | | | M. McDonnell(1) | | $ | 850,000 | | | | 75 | % | | | 95 | % | | | 115 | % | | $ | 263,322 | | | | | | | | A. Sandrock | | $ | 901,680 | | | | 75 | % | | | 95 | % | | | 112 | % | | $ | 719,541 | | | | | | | | S. Alexander | | $ | 814,168 | | | | 70 | % | | | 95 | % | | | 112 | % | | $ | 606,392 | | | | | | | | C. Guindo | | $ | 567,100 | | | | 70 | % | | | 95 | % | | | 140 | % | | $ | 527,970 | | | | | | | | J. Capello(2) | | $ | 811,125 | | | | 75 | % | | | n/a | | | | n/a | | | | n/a | |
Notes to the 2020 Annual Bonus Plan Awards Table Revenues
| | | 28%
12%
| | |
| 2018
2019
2020
2018
2019
2020
|
| | $ 2.9B
Target set at
beginning of 2019 Target set at
beginning of 2020 $ 12.8B
Target set at
beginning of 2019 Target set at
beginning of 2020
| | $ 4.0B(1)
TBDTBD
$ 13.4B(2)
TBD
TBD
| (1) | Mr. McDonnell was appointed as our Executive Vice President and Chief Financial Officer effective August 15, 2020. Mr. McDonnell’s award under our 2020 Annual Bonus Plan was based on his prorated salary for 2020. |
| (2) | Mr. Capello ceased to be employed by the Company during 2020 and, as a result, was ineligible for a payout under our 2020 annual bonus plan. |
| | | | | | | | | 48 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Long-Term Incentives Below is a summary of the types of annual LTI awards granted to our NEOs for 2020.* | | | | | | | | | | | Terms | | Performance Stock Units (PSUs) | | Market Share Units (MSUs) | Proportion of Annual Target Value | | 50% | | 50% | | Settlement | | 60% Stock Settled | | 40% Cash Settled | | 100% Stock Settled | | Performance Period(s) | | 3 years (2020-2022) | | 1 year (each of 2020, 2021, 2022) | | 1 year, 2 years, 3 years (from grant date) | Metrics and Weighting | | Pipeline Milestone Performance: 60% | | Adjusted Free Cash Flow: 28% Revenue: 12% | | Stock Price: 100% | Threshold / Maximum Payout (% of Target Award) | | 50% / 200% | | 50% / 200% | | 50% / 200% | | Vesting | | 3-year Cliff Vesting | | 3-year Cliff Vesting | | Annual Ratable Vesting over 3 years (1/3 per year) |
* Does not include the sign-on LTI awards granted to Mr. McDonnell in connection with his hire. All annual LTI awards granted to our executive officers are performance-based and designed to reward long-term Company performance. Our executive annual LTI program for 2020 consisted of PSUs and MSUs, with the annual LTI total target grant value of awards being split evenly between PSUs and MSUs. The PSUs we granted to executive officers are performance-based RSUs that are settled, as applicable, in cash and shares of our common stock. The MSUs we granted to executive officers are performance-based RSUs that are settled in shares of our common stock. The performance conditions applicable to these PSUs and MSUs are described in further detail below. Our annual LTI target grant values are determined based on an executive’s individual performance, potential future contributions and market competitiveness as well as other factors. In determining the annual LTI target grant value, our C&MD Committee reviews LTI awards of our peer group and, in certain circumstances, the broader market, and also reviews the total compensation of our executive officers against our peer group and, in certain circumstances, the broader market. In general, we have a heavier weighting in our executive compensation mix towards LTI awards in order to better align the interests of our executives with those of our stockholders. On average, annual LTI target grant values for our NEOs position their total compensation at or around the median values of our peer group in cases where there are comparable positions at the peer companies. We have an established annual LTI grant practice where LTI grants are made following the completion of our internal performance reviews of our executive officers as well as our external market review of equity practices of our peer group and the broader market, including the data from the Willis Towers Watson survey described above. Since 2004 we have made our annual LTI grants in February of each year following our annual earnings release. We generally grant time-based RSUs in lieu of PSUs at the time an executive is hired if employment commences after June 30th with three-year cliff-vesting to mirror the vesting terms of the PSUs. These grants are generally granted on the first trading day of the month following the date of hire. From time to time, we also grant time-based RSUs to recognize extraordinary contributions to the Company or for transition or retention purposes. In connection with Mr. McDonnell’s hire in August 2020, we granted him time-based RSUs, in addition to MSUs. | | | | | | | | | 49 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
In 2020 the LTI grant values for our NEOs were as follows: | | | | | | | | | Name | | Annual LTI Grant Value | | | | | | M. Vounatsos | | $12,500,000 | | | | | | M. McDonnell(1) | | $ 4,500,000 | | | | | | A. Sandrock | | $ 4,000,000 | | | | | | S. Alexander | | $ 3,200,000 | | | | | | C. Guindo | | $ 3,800,000 | | | | | | J. Capello(2) | | $ 3,500,000 | | |
Notes to the 2020 LTI Awards Table | (1) | Mr. McDonnell joined Biogen after the annual LTI awards were granted. Mr. McDonnell received a new hire grant in September 2020, which consisted of RSUs and MSUs with an aggregate grant date value of $4.5 million. The RSUs will vest in three annual installments beginning on the first anniversary of the grant date. |
| (2) | Mr. Capello forfeited his 2020 grants in connection with the termination of his employment. |
The actual value that will be realized from PSU awards depends on the degree of achievement of performance goals, with 60% of the PSUs (based on the grant date target value) settled in shares of our common stock based upon achievement of cumulative three-year pipeline milestone goals and the remaining 40% of the PSUs settled in cash based upon the achievement of two annual financial goals that are determined at the beginning of each relevant year. The actual value that will be realized from MSU awards depends on our 30-day average common stock price growth between the grant date and each of the dates such awards vest. If the vesting price is higher than the grant price, the number of shares that vest will be increased and in contrast, if the vesting price is lower than the grant price, the number of shares that vest will be decreased. Our common stock price is influenced by the Company’s performance as well as external market factors. 2020 PSUs PSUs comprised 50% of our NEO’s target annual LTI awards for 2020. PSUs are performance-based RSUs that have three-year cliff vesting in furtherance of the Company’s long-term pay-for-performance philosophy and to encourage employee retention. PSUs align executive compensation to Company goals through performance against pipeline milestone performance goals. The actual value (if any) of PSUs will not be realized by the NEOs until the three-year period ends and then only if the applicable performance goals are achieved. For our 2020 PSU awards, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock based on achievement of pipeline performance goals over a three-year performance period (2020 to 2022) (the 2020 Stock-Settled PSUs) and continued employment through the vesting date. The remaining 40% of the PSUs will be settled in cash based on the achievement of three one-year financial goals (2020, 2021 and 2022) (the 2021 Cash-Settled PSUs) and continued employment through the vesting date. Our 2020 PSU awards are scheduled to vest in February 2023. For our 2020 PSU awards, the number of PSUs earned at the end of the three-year performance period will be determined as follows: 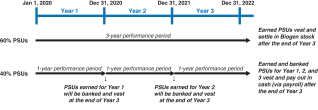
In designing our 2020 PSU LTI program, our C&MD Committee acknowledged the need to balance driving long-term performance and investing for the future with achieving key milestones along the way. Cash-based payments are primarily aligned with and reward more recent performance, while equity awards encourage our executives to continue to deliver results over a longer period of time and also serve as a retention tool. Our C&MD Committee determined that delivering a substantial portion of compensation in the form of equity awards with longer-term goals would further align our executive officers’ interests with those of Biogen’s stockholders in creating long-term stockholder value. 2019 PSUs For our 2019 PSU awards, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock based on achievement of financial and pipeline milestone performance goals over a three-year performance period (2019 to 2021) (the 2019 Stock-Settled PSUs) and continued employment through the vesting date. The remaining 40% of the PSUs will be settled in cash based on the achievement of three one-year financial goals (2019, 2020 and 2021) (the 2019 Cash-Settled PSUs) and continued employment through the vesting date. Our 2019 PSU awards are scheduled to vest in February 2022. | | | | | | | | | 50 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
2018 PSUs For our 2018 PSU awards, 60% of the PSUs (based on the grant date target value) were settled in shares of our common stock based on achievement of financial and pipeline milestone performance goals over a three-year performance period (2018 to 2020) (the 2018 Stock-Settled PSUs). The remaining 40% of the PSUs were settled in cash based on the achievement of three one-year financial goals (2018, 2019 and 2020) (the 2018 Cash-Settled PSUs) and continued employment through the vesting date. Our 2018 PSU awards vested on February 12, 2021. The performance metrics and weightings, and the degree to which we achieved the performance goals, for the 2018 Stock-Settled PSUs and the 2018 Cash-Settled PSUs are described in further detail below. 2020 PSU Awards Table Set forth below is a summary of the performance metrics and weightings that our C&MD Committee established for our 2020 PSU awards and the degree to which we achieved the performance goals for the 2020 tranche of the 2020 Cash-Settled PSUs. At the time our C&MD Committee established the performance goals for the 2020 tranche of the 2020 Cash-Settled PSUs in the beginning of the year, we did not know what impact, including the timing, that generic competition for TECFIDERA would have on our 2020 financial results as it was dependent on a number of factors beyond our control, including decisions in the patent infringement proceedings relating to TECFIDERA Orange-Book listed patents pursuant to the Hatch-Waxman Act in West Virginia and Delaware. Due to this uncertainty, our C&MD Committee exercised its discretion to make certain adjustments to the results to take into account items that were not originally contemplated, or whose magnitude or timing were uncertain, at the time the performance goals for the 2020 tranche of the 2020 Cash-Settled PSUs were originally determined. In particular, the entry of multiple TECFIDERA generic entrants in the U.S. with deeply discounted prices compared to TECFIDERA had real and negative impacts on our financial results. Our C&MD Committee believed these adjustments were appropriate because the items were beyond the control of management, were not contemplated and/or could not be quantified due to uncertainty regarding magnitude and timing when the performance goals for the 2020 tranche of the 2020 Cash-Settled PSUs were originally set. Our C&MD Committee also believed that adjustments were necessary to appropriately motivate and reward employees for their performance during a challenging year in which we continued to perform well despite the challenges that we faced. Based on our overall performance, and following these adjustments, the multiplier for the 2020 tranche of the 2020 Cash-Settled PSUs was 100%. However, notwithstanding the attainment of these performance metrics and the strength of management’s performance, our C&MD Committee believed it was important to hold the members of our Executive Committee, which includes all of our NEOs, accountable for the Company’s overall financial results and business performance. As a result, our C&MD Committee exercised its discretion and decreased the multiplier for the 2020 tranche of the 2020 Cash-Settled PSUs for the members of our Executive Committee, including all of our NEOs, by 10%, which resulted in a multiplier for our NEOs of 90%. | | | | | | | | | 51 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
We utilized the same performance metrics for the 2020 (i.e., second) tranche of the 2019 Cash-Settled PSUs and the 2020 (i.e., third) tranche of the 2018 Cash-Settled PSUs and, therefore, the multiplier for the 2020 tranche of the 2019 Cash-Settled PSUs and the 2018 Cash-Settled PSUs for the NEOs was also 90% for our NEOs. | | | | | | | | | | | | | | | | | | | | | | | | Percentage of
PSU Award | | Percentage of
PSU Target
Value / Total
LTI Target
Value | | Performance Metrics | | Performance
Metrics
Weight | | | Performance
Period | | | Target Performance | | Actual
Performance | Stock- Settled: 60% | | 60% / 30% | | Pipeline Milestone Performance | | | 60% | | | | 2020-2022 | | | Specific goals are not disclosed for competitive reasons | Cash- Settled: 40% | | 40% / 20% | | Adjusted Free Cash Flow Revenue | | | 28% 12% | | |
| 2020
2021 2022 2020 2021 2022 |
| | $ 6.3B Target set at beginning of 2021 Target set at beginning of 2022 $ 14.1B Target set at beginning of 2021 Target set at beginning of 2022 | | $ 6.3B(1)
TBDTBD $ 13.9B(2)
TBD TBD |
Notes to the 2020 PSU Awards Table | (1) | This financial measure was based on our Non-GAAP free cash flow, as adjusted to exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020, the addition of $177.0 million related to a one-time license payment from a contract manufacturing customer and $52.0 million related to share repurchases in 2020 under our 2020 Share Repurchase Program, our December 2019 Share Repurchase Program and our March 2019 Share Repurchase Program. These items were either not originally contemplated or their magnitude or timing was uncertain at the time the Company performance goals were originally established. |
| (2) | This financial measure was based on our publicly reported revenue of $13.4 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations and exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020. |
The 2020 Stock-Settled PSUs metric, which was approved by our C&MD Committee, was the achievement of a pipeline milestone performance for the three-year period of 2020 through 2022. Pipeline milestone performance over the three-year period of 2020 through 2022 was selected to drive our long-term strategic direction and stockholder value creation through our pipeline progress and development. The 2020 Cash-Settled PSUs financial metrics are adjusted free cash flow and revenue. At the beginning of each year during the performance period for our 2020 Cash-Settled PSU awards, our C&MD Committee will approve the targets for each of these financial metrics for such year. Our C&MD Committee decided that because of the nature of our business, in which operating metrics can potentially be impacted positively or negatively by events outside of the control of executives, the design of the PSU program would be based, in part, on the use of three one-year financial goals. Our C&MD Committee views free cash flow as a critical measure to align the interests of management with those | | of our stockholders as it reflects the net cash flow available to the 2018 PSU Awards TableCompany to pursue opportunities and investments that enhance stockholder value. As such, a cash flow performance goal encourages management to optimize capital expenditures, invest prudently in high return projects and optimize working capital. |
We selected revenue as a performance measure to further reinforce the importance of achieving and exceeding our revenue goal and to provide further incentive to achieve such goal and its tie to stockholder value creation through the longer-term vesting requirements associated with the goal. In order to further motivate our executives to drive the organization toward the achievement and potential over-achievement of these goals, we provide for a maximum payout opportunity of 200% for our PSU awards. Participants may ultimately earn between 0% and 200% of the target number of PSUs granted based on the degree of actual performance goal achievement, generally subject to continued service with the Company. | | | | | | | | | 52 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
(1) | This financial measure was based on ourNon-GAAP free cash flows, as adjusted to add back $256 million to reflect the cash impact of additional research and development expense recognized in 2018 resulting from the 2018 Ionis Agreement, $16 million to neutralize the unfavorable cash impact of the worldwide withdrawal of ZINBRYTA and $33 million related to higher than originally contemplated stock repurchases in 2018, partially offset by the subtraction of $235
2018 PSU Award Payout Set forth below is a summary of the performance metrics and weightings that our C&MD Committee established for our 2018 PSU awards and the degree to which we achieved the performance goals for the 2018 Stock-Settled PSUs and the 2018 Cash-Settled PSUs. 2018 Stock-Settled PSUs | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Performance Target | | | | | | | | | | | | | | | | Performance Metrics | | Performance
Period | | | Threshold | | | Target | | | Max | | | Results | | Weight | | | Payout | | Adjusted Non-GAAP diluted EPS | | | 2018-2020 | | | $ | 26.98 | | | $ | 28.11 | | | $ | 29.76 | | | $30.79(1) | | | 30 | % | | | 200.0 | % | Pipeline Milestone Performance | | | 2018-2020 | | |
| Specific goals are not disclosed
for competitive reasons |
| | Met
Goal(2) | | | 30 | % | | | 100.0 | % | 2018 Stock-Settled PSU Multiplier | | | | 150.0 | % |
Notes to the 2018 Stock-Settled PSUs Table | (1) | These financial measures were based on our publicly announced Non-GAAP diluted EPS of $33.70, as adjusted as follows: the addition of $0.80 to exclude the impact of business development transactions and $2.09 to exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020, partially offset by the subtraction of $5.80 to exclude the impact of share repurchases. These items were either not originally contemplated or their magnitude or timing was uncertain at the time the Company performance goals were originally established. |
| (2) | The Company continued to expand and re-shape its pipeline of pre-clinical and clinical stage programs through the advancement of internal programs, external business development activities and meeting expectations with respect to the level of confidence in and momentum of its clinical stage portfolio. Specific details are not disclosed for competitive reasons. |
2018 Cash-Settled PSUs | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Performance Target | | | | | | | | | | | | | | | | Performance Metrics | | Performance
Period | | | Threshold | | | Target | | | Max | | | Results | | Weight | | | Multiplier | | Adjusted Free Cash Flow | | | 2018 | | | $ | 2.7B | | | $ | 2.9B | | | $ | 3.3B | | | $4.0B(1) | | | 28 | % | | | 200.0 | % | Revenue | | | 2018 | | | $ | 12.3B | | | $ | 12.8B | | | $ | 13.5B | | | $13.4B(2) | | | 12 | % | | | 172.6 | % | 2018 Tranche of 2018 Cash-Settled PSU Multiplier | | | | 192.0 | %* | Adjusted Free Cash Flow | | | 2019 | | | $ | 4.9B | | | $ | 5.5B | | | $ | 6.3B | | | $6.0B(3) | | | 28 | % | | | 165.9 | % | Revenue | | | 2019 | | | $ | 13.0B | | | $ | 13.7B | | | $ | 14.6B | | | $14.4B(4) | | | 12 | % | | | 149.5 | % | 2019 Tranche of 2018 Cash-Settled PSU Multiplier | | | | 161.0 | %* | Adjusted Free Cash Flow | | | 2020 | | | $ | 5.7B | | | $ | 6.3B | | | $ | 7.0B | | | $6.3B(5) | | | 28 | % | | | 104.5 | % | Revenue | | | 2020 | | | $ | 13.3B | | | $ | 14.1B | | | $ | 15.0B | | | $13.9B(6) | | | 12 | % | | | 90.9 | % | 2020 Tranche of 2018 Cash-Settled PSU Multiplier | | | | 100.0 | % | Adjusted 2020 Tranche of 2018 Cash-Settled PSU Multiplier for NEOs | | | | 90.0 | %*(7) |
* Numbers may not recalculate due to rounding. Notes to the 2018 Cash-Settled PSUs Table | (1) | This financial measure was based on our Non-GAAP free cash flow, as adjusted to add back $256.0 million to reflect the cash impact of additional research and development expense recognized in 2018 resulting from our 2018 agreement with Ionis Pharmaceuticals, Inc. to develop novel ASO drug candidates for a broad range of neurological diseases, $16.0 million to neutralize the unfavorable cash impact of the worldwide withdrawal of ZINBRYTA and $33.0 million related to higher than originally contemplated stock repurchases in 2018, partially offset by the subtraction of $235.0 million to reflect tax payments made in connection with tax reform, as these charges were not originally contemplated at the time these performance goals were determined. |
| (2) | (2) | This financial measure was based on our publicly reported revenuesThis financial measure was based on our publicly reported revenue of $13.5 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations.
|
| | | | | | | | | The 2018 Stock-Settled PSUs53
| | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| (3) | This financial measure was based on our Non-GAAP free cash flow, as adjusted to subtract $170.0 million for the decline in operating taxes due to the change in the Company’s tax profile, $58.0 million for cost savings due to the termination of certain clinical programs in 2019 and $78.0 million to remove the favorable impact of the launch delay of rituximab biosimilars, partially offset by the addition of $33.0 million to reflect the impact of our acquisition of Nightstar Therapeutics plc and $50.0 million related to higher than originally contemplated stock repurchases in 2019, as none of these items were originally contemplated at the time these performance goals were determined. |
| (4) | This financial measure was based on our publicly reported revenue of $14.4 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations. |
| (5) | This financial measure was based on our Non-GAAP free cash flow, as adjusted to exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020, the addition of $177.0 million related to a one-time license payment from a contract manufacturing customer and $52.0 million related to share repurchases in 2020 under our 2020 Share Repurchase Program, our December 2019 Share Repurchase Program and our March 2019 Share Repurchase Program. These items were either not originally contemplated or their magnitude or timing was uncertain at the time the Company performance goals were originally established. |
| (6) | This financial measure was based on our publicly reported revenue of $13.4 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations and exclude the adverse impact of multiple TECFIDERA generic entrants that entered the U.S. market in 2020. |
| (7) | Notwithstanding the attainment of these performance metrics were approved byand the strength of management’s performance, our C&MD Committee with equal weighting assignedbelieved it was important to each metric. The two metrics selected werehold the achievementmembers of a cumulative three-year adjustedNon-GAAP diluted EPS and pipeline milestone performance, in each case,our Executive Committee, which includes all of our NEOs, accountable for the three-year period of 2018 through 2020. AdjustedNon-GAAP diluted EPS measured at the end of three-year performance period was selected to reinforce the importance of achieving long-termCompany’s overall financial results and operationalbusiness performance. Our C&MD Committee believes that adjustedNon-GAAP diluted EPS isAs a transparent, operations-based measure.
Pipeline milestone performance over the three-year period of 2018 through 2020 was selected to drive our long-term strategic direction and stockholder value creation through our pipeline progress.
The 2018 Cash-Settled PSUs financial metrics are adjusted free cash flows and revenues. At the beginning of each year during the performance period for our 2018 PSU awards,
result, our C&MD Committee will approveexercised its discretion and decreased the targetsmultiplier for each of these financial metrics for such year. Our C&MD Committee decided that becausethe 2018 tranche of the nature2020 Cash-Settled PSUs for the members of our business, in which operating metrics can potentially be impacted positively or negatively by events outside of the control of executives, the design of the PSU program would be based, in part, on the use of threeone-year financial goals. Our C&MDExecutive Committee, views free cash flow as a critical measure to align the interests of management with thoseincluding all of our stockholders as it reflects the net cash flows available to the Company to pursue opportunities that enhance stockholder value. As such,NEOs, by 10%, which resulted in a cash flow performance goal encourages management to optimize capital expenditures, invest prudently in high return projects and optimize working capital.multiplier for our NEOs of 90%.
|
2018 PSUs Payout The final payouts under our 2018 Stock-Settled PSUs and 2018 Cash-Settled PSUs were as follows: | | | | | | | | | | | | | | | | | | | | | | | Name | | Target 2018 Stock-
Settled PSU
Award at Grant (#) | | | Actual 2018
Stock-Settled
PSU Award
Earned (#) | | | Target 2018
Cash-Settled
PSU Award
at Grant ($) | | | Actual 2018
Cash-Settled
PSU Award
Earned ($) | | M. Vounatsos | | | 10,895 | | | | 16,343 | | | $ | 2,300,000 | | | $ | 2,912,816 | | M. McDonnell | | | — | | | | — | | | | — | | | | — | | A. Sandrock | | | 2,485 | | | | 3,728 | | | $ | 525,000 | | | $ | 665,229 | | S. Alexander | | | 3,030 | | | | 4,545 | | | $ | 640,000 | | | $ | 809,617 | | C. Guindo | | | — | | | | — | | | | — | | | | — | | J. Capello | | | — | | | | — | | | | — | | | | — | |
2020 MSUs MSUs comprised 50% of our executives’ target LTI for 2020. MSUs are performance-based RSUs that are earned based on our common stock price performance from the date of grant to each of the three annual vesting dates. Each annual tranche is assessed against our stock price on the original grant date, such that the awards vesting in 2021, 2022 and 2023 will be assessed against the 2020 grant date stock price, thereby aligning long-term executive interests with those of our stockholders. On each vesting date, the performance multiplier is determined based on the stock price growth measured from the grant date to such vesting date using the average closing stock price for the 30 calendar days following and including the grant date and 30 calendar days prior to and including such vesting date for MSUs granted in 2020. Participants may ultimately earn between 0% and 200% of the target number of MSUs awarded based on our actual stock performance. The maximum payout percentage of MSUs granted in 2020 is consistent with those granted in 2019 (200%). Once the performance multiplier is determined, it is applied to the target number of MSUs granted to each executive and can increase or decrease the overall number of MSUs earned based on our stock price performance. | | | | | | | | | 54 | | 
| |  |
We selected revenues as a performance measure to reinforce the importance of achieving and exceeding our revenue goal and to provide further incentive to achieve such goal.
| | | | | 48 | |  | |  |
| | | 5 | | 6 | | Executive Compensation Matters (continued) |
In order to further motivate our executives to drive the organization toward the achievement of these goals, we provide for a maximum payout of 200% for our 2018 PSU awards. Participants may ultimately earn between 0% and 200% of the target number of PSUs granted based on the degree of actual performance goal achievement, generally subject to continued service with the Company.
2018 MSUs
MSUs comprised 50% of our executives’ target LTI for 2018. MSUs are performance-based RSUs that are earned based on our common stock price performance from the date of grant to each of the three annual vesting dates. On each vesting date, the performance multiplier is derived based on the stock price growth measured from the grant date to such vesting date using the average closing stock price for the 30 calendar days following and including the grant date and 30 calendar days prior to and including such vesting date for MSUs granted in 2018.
Participants may ultimately earn between 0% and 200% of the target number of MSUs awarded based on actual stock performance. The maximum payout percentage of MSUs granted in 2018 is consistent with those granted in 2017 (200%). Once the performance multiplier is determined, it is applied to the target number of MSUs granted to each executive and can increase or decrease the overall number of MSUs earned based on stock price performance.
MSU Illustration 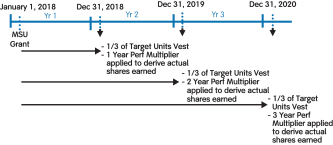 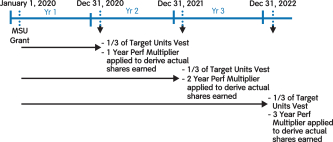
The three-year service vesting period ties executive compensation directly to our common stock price performance, as both the MSUs earned and the value actually received in respect of MSUs are dependent on the performance of our common stock over the three-year vesting period. On each vesting date, the earned MSUs are settled in shares of our common stock. MSUs directly align long-term interests of our executives with stockholders, as the payout is directly tied to stock price performance. The following table shows the vesting date, performance period and performance multiplier applied for MSUs vesting in 20182020 and 2019:2021: | | | | | | | | | | | | | | | | Grant Date | | Vesting Date | | Performance Period | | | Performance Multiplier | | | | | | 2/20182020 | | 2/20192021 | | | 1 year | | | | 114%85% | | | | | | 2/20172019 | | 2/20192021 | | | 2 years | | | | 124%84% | | | | 2/20182020 | | | 1 year | | | | 126%92% | | | | | | 2/20162018 | | 2/20192021 | | | 3 years | | | | 134%94% | | | | 2/20182020 | | | 2 years | | | | 132%103% | |
2018One-Time Transition AwardsTotal Target Direct Compensation and Realizable Pay
As part of its review of our 2018 LTI program change and transition plan,NEOs’ compensation, our C&MD Committee decidedconsiders total target direct compensation as well as realizable pay. Total target direct compensation refers to grantone-time transition awardsthe sum of salary, target bonus and target LTI. Realizable pay refers to the sum of salary, actual bonus and the tracked potential payout value of LTI, including performance criteria and stock price. Other elements such as changes in pension value and nonqualified deferred compensation earnings, shown in the formSummary Compensation Table, are not considered in these analyses because they are program-based benefits whose plan designs are not typically subject to annual change. Our C&MD Committee believes these assessments can be used to better align pay with market by focusing on core compensation elements as well as the intended target value and actual value of time-based RSUsthese awards consistent with our pay for performance philosophy. For example, Mr. Vounatsos’ 2020 total target direct compensation was $16.25 million, comprised of $1.5 million base salary, $2.25 million target bonus (150% of base salary) and $12.5 million target LTI. As of December 31, 2020, Mr. Vounatsos’ realizable pay was calculated at $13.18 million, which reflects 81% of the target intended value to be delivered, and is comprised of $1.5 million base salary, $2.57 million actual bonus payout and $9.12 million LTI tracking value (numbers may not foot based on rounding). The LTI value in February 2018 to certain executive officers, excluding Messrs. Vounatsos and Capello, which vest over atwo-year period, with 33% vesting on the first anniversarySummary Compensation Table differs from our C&MD Committee approved target value for 2020 because of the valuation methodology used for accounting purposes in reporting the grant date fair value of equity awards. This valuation is done in accordance with ASC 718. Because incentive compensation makes up such a large portion of compensation for our NEOs, we believe tracking the actual payout and 67% vesting on the second anniversarypotential realizable value of the grant date. These awards were intended to help mitigate the impact on executives’ compensation and cash flow disruption due to the program changes, including the change to the three-year cliff vesting schedule applied to the PSU awards discussed above compared to the annual installment vesting over three years that appliedintended target value to the CSPUs that we previously granted. In 2018 theone-time transition awards of RSUs forbe delivered can help ensure our NEOs were as follows:performance-based incentives and overall compensation are aligned with stockholders.
| | | | | Name | | Grant
Date Value
| | | | | M. Vounatsos
| | n/a | | | | | J. Capello
| | n/a | | | | | M. Ehlers
| | $ 1,500,000 | | | | | S. Alexander
| | $ 1,280,000 | | | | | P. McKenzie
| | $ 1,200,000 | | |
Retirement Plans We maintain a Supplemental Savings Plan (SSP), which is anon-qualified deferred compensation plan covering our executive officers and other eligible employees in the U.S. We offer the SSP as part of the retirement savings component of our benefits program. We designed the SSP to be competitive with thenon-qualified deferred compensation plans offered by companies in our peer group at that time. Details of the SSP are discussed under the heading “2018“2020 Non-Qualified Deferred Compensation” below. | | | | | 49 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
Other Benefits In addition to eligibility for the benefit programs generally provided to all employees, such as our employee stock purchase plan, 401(k) plan and medical, dental, vision, life and disability insurance, we provide certain supplemental benefits to our executives. These benefits include: Life Insurance All of our U.S. executives, including our NEOs, receive Company-paid term life insurance equal to three times their annual base salary, up to a maximum benefit amount. In 20182020 the maximum benefit amount for the CEO was $1.5 million and was $2.25 million for the other NEOs. Employees who are not executives receive Company-paid term life insurance equal to two times their annual base salary. The additional value of Company-provided life | | | | | | | | | 55 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
insurance for our executive officers reflects competitive practices and is consistent with our philosophy to provide appropriate levels of financial security for our employees based on their positions within the Company. The cost of Company-paid life insurance in excess of a $50,000 insurance level is taxable income to U.S. employees and is not grossed up by the Company. Executive Physicals, Tax Preparation, Financial and Estate Planning Our executive officers, other than our CEO, are eligible for reimbursement of expenses incurred for tax preparation and financial and estate planning services as well as the purchase of tax preparation and financial planning software, subject to annual expense limits of $7,500 for Executive Vice Presidents. Such reimbursements are taxable income to our executives and are not grossed up. All of our executive officers, including our CEO, are eligible for reimbursement for the cost of their executive physicals, subject to the annual expense limits noted above of $7,500 for our Executive Vice Presidents and CEO. This benefit provides our executives with additional flexibility to proactively manage their health and wellness. Relocation Expenses Under our Executive Relocation Policy, we will, in certain circumstances, provide relocation benefits when employees first join us. Post-Termination Compensation and Benefits We provide severance benefits to all of our executive officers if they aretheir employment is terminated without cause or in certain other circumstances. The terms of these arrangements and the amounts payable under them are described below for each NEO under the heading “Potential Payments Upon Termination or Change in Control.” We provide these benefits because we believe that severance protection is necessary to help our executives maintain their focus on the best interests of the Company when providing advice to the Company and when making strategic decisions about a potential corporate transaction or change in control, and further encourages effective leadership in the closing and integration of significant transactions affecting the Company. Stock Ownership Guidelines We maintain stock ownership guidelines for our executive officers to strengthen and reinforce the link our compensation programs create between our executives and our stockholders. A summary of our stock ownership guidelines is set forth below. | | | | | | Level | | Number of Shares Equal in Value to: | | | CEO | | 6x base salary | | | | | Executive Vice Presidents | | 3x base salary | | |
Executive officers have five years from their initial appointment to meet thethis requirement. In the event the requirement is not met within that time, 100% of vested shares received in respect of LTI awards are required to be held until the requirement is satisfied. Only stock owned outright or otherwiseand underlying vested or earned performance-based sharesequity awards is credited toward the stock ownership requirement. Shares underlying unvested or unearned performance-based sharesequity awards are not included in the calculation. All of our executive officers currently meet the stock ownership requirement or are still within the five-year period to meet such requirement. Recoupment of Compensation We may recover compensation from our employees, including our executive officers, who engage in detrimental or competitive activity. Detrimental activity includes any action or failure to act that constitutes financial malfeasance that is materially injurious to the Company, violates our Code of Business Conduct (Values in Action), results in a restatement of our earnings or financial results or results in a violation or breach of law or contract. Competitive activity includes any action or failure to act that violatesnon-disclosure,non-competition and/ornon-solicitation agreements. Our 20082020 Performance-Based Management Incentive Plan | | | | | 50 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
allows for the forfeiture and/or repayment of cash-based awards, and our 2008 Omnibus Equity Plan and our 2017 Omnibus Equity Plan each allow for the cancellation of LTI awards in these circumstances as well as the forfeiture of stock or cash acquired upon vesting or sale of LTI awards. In addition, cashsign-on bonuses paid to our NEOs may be subject to repayment if the NEO voluntarily resigns from the Company or if his or her employment is terminated by the Company in certain circumstances. | | | | | | | | | 56 | |  �� ��
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Insider Trading, Hedging and Pledging Policy Prohibitions We maintain a Global Insider Trading and Information Policy that prohibits our employees, officers, temporary staff and directors, members of their immediate family and family trusts (or similar entities) controlled by or benefitting such persons from, among other things, engaging in hedging or derivative or similar transactions with respect to the Company’s equity securities, including, purchases or sales of puts and calls, options, forward contracts, put and call collars, equity or performance swap or exchange fund agreements, or any similar agreements or arrangements, purchasing Company stock on margin, borrowing against any account in which Company securities are held, pledging Company securities as collateral for a loan or engaging in short sales of the Company’s securities. No categories of hedging transactions are specifically permitted by our Global Insider Trading and Information Policy and those that are specifically prohibited are noted above. Tax-Deductibility of Compensation Section 162(m) of the Internal Revenue Code generally limits the amount a company may deduct for compensation in excess of $1$1.0 million paid to certain “covered employees,” subject to certain transition relief applicable to certain arrangements in place as of November 2, 2017, and not materially modified after such date. Our C&MD Committee regularly reviews the provisions of our plans and programs, works with its independent compensation consultant and reviews and considers, among other things, the tax deductibility of compensation payments. Our C&MD Committee, however, believes that compensation programs that attract, retain and reward executive talent and achievement are necessary for our success and, therefore, are in the best interests of the Company and our stockholders and that, in establishing the cash and equity incentive compensation programs for the Company’s executive officers,without regard to the potential deductibility of the compensation payable under such programs should only be one of a number of relevant factors taken into consideration.programs. Consequently, our C&MD Committee may pay or provide, and has paid or provided, compensation that is not tax deductible in whole or in part. Compensation Committee Report The Compensation and Management Development Committee furnishes the following report: The Compensation and Management Development Committee has reviewed and discussed the Compensation Discussion and Analysis with Biogen management. Based on this review and discussion, the Compensation and Management Development Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement. Submitted by, Robert W. Pangia (Chair) Richard C. Mulligan Eric K. Rowinsky
Lynn SchenkBrian S. Posner
| | | | | | | 51 | | 57 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
Summary Compensation Table The following table shows the compensation paid to or earned by our NEOs during the years ended December 31, 2018,2020, December 31, 2017,2019, and December 31, 2016,2018, for the year(s) in which they were a named executive officer. | | Name and Principal Position (a) | | Year (b) | | Salary (c) | | Bonus(1) (d) | | Stock Awards(2) (e) | | Non-Equity Incentive Plan Compensation(3) (f) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings(4) (g) | | All Other Compensation(5) (h) | | Total (i) | | Year (b) | | Salary (c) | | Bonus(1) (d) | | Stock Awards(2) (e) | | Non-Equity Incentive Plan Compensation(3) (f) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings(4) (g) | | All Other Compensation(5) (h) | | Total (i) | | Michel Vounatsos(6) | | | 2018 | | | $ | 1,276,923 | | | | — | | | $ | 11,064,897 | | | | $3,337,880 | | | | $ 80,663 | | | | $408,283 | | | $ | 16,168,646 | | | | | | | | | | | | | | | | | | | | | Michel Vounatsos | | | 2020 | | | $ | 1,488,462 | | | | — | | | $ | 13,887,064 | | | $2,565,000 | | | $ 264,358 | | | $ 454,945 | | | $ | 18,659,829 | | Chief Executive Officer | | | 2017 | | | $ | 1,087,885 | | | | — | | | $ | 9,868,540 | | | | $2,502,500 | | | | $ 18,881 | | | | $186,567 | | | $ | 13,664,373 | | | 2019 | | | $ | 1,388,461 | | | | — | | | $ | 12,352,030 | | | $3,884,000 | | | $ 85,667 | | | $ 449,700 | | | $ | 18,159,858 | | | | | 2016 | | | $ | 519,231 | | | $ | 1,500,000 | | | $ | 3,151,199 | | | | $ 447,799 | | | | $ 1,598 | | | | $181,568 | | | $ | 5,801,395 | | | 2018 | | | $ | 1,276,923 | | | | — | | | $ | 11,064,897 | | | $3,337,880 | | | $ 80,663 | | | $ 408,283 | | | $ | 16,168,646 | | Jeffrey D. Capello(7) | | | 2018 | | | $ | 750,000 | | | | — | | | $ | 2,889,224 | | | | $ 790,913 | | | | — | | | | $ 46,582 | | | $ | 4,476,719 | | | Executive Vice President | | | 2017 | | | $ | 28,846 | | | $ | 520,000 | | | | — | | | | — | | | | — | | | | $ 132 | | | $ | 548,978 | | | | and Chief Financial Officer | | | Michael D. Ehlers | | | 2018 | | | $ | 829,511 | | | | — | | | $ | 5,107,535 | | | | $ 917,837 | | | | $ 5,258 | | | | $186,510 | | | $ | 7,046,651 | | | Michael R. McDonnell(6) | | | | 2020 | | | $ | 294,231 | | | $ | 1,000,000 | | | $ | 5,012,308 | | | | $ 263,322 | | | | — | | | | $ 5,696 | | | $ | 6,575,557 | | | Executive Vice President and Chief Financial Officer | | | | | | | | | | | | | | | | | | Alfred W. Sandrock, Jr. | | | | 2020 | | | $ | 890,310 | | | | — | | | $ | 4,331,654 | | | | $ 719,541 | | | | $ 138,444 | | | | $ 147,020 | | | $ | 6,226,969 | | Executive Vice President, | | | 2017 | | | $ | 792,139 | | | | — | | | $ | 3,157,338 | | | | $1,012,034 | | | | $ 1,799 | | | | $101,671 | | | $ | 5,064,981 | | | 2019 | | | $ | 798,723 | | | | — | | | $ | 3,360,682 | | | $ 893,038 | | | $ 78,506 | | | $ 181,616 | | | $ | 5,312,565 | | Research & Development | | | 2016 | | | $ | 491,827 | | | $ | 1,170,177 | | | $ | 3,410,650 | | | | $ 425,062 | | | | $ 155 | | | | $ 14,665 | | | $ | 5,512,536 | | | | | | | | | | | | | | | | | | Susan H. Alexander | | | 2018 | | | $ | 746,254 | | | | — | | | $ | 4,359,590 | | | | $ 858,744 | | | | $188,056 | | | | $201,140 | | | $ | 6,353,784 | | | | 2020 | | | $ | 809,695 | | | | — | | | $ | 3,583,281 | | | | $ 606,392 | | | | $ 311,416 | | | | $ 159,261 | | | $ | 5,470,045 | | Executive Vice President, | | | 2017 | | | $ | 720,630 | | | | — | | | $ | 3,157,338 | | | | $ 856,305 | | | | $148,961 | | | | $178,008 | | | $ | 5,061,242 | | | 2019 | | | $ | 772,373 | | | | — | | | $ | 3,302,619 | | | $1,033,451 | | | $ 164,782 | | | $ 181,785 | | | $ | 5,455,010 | | Chief Legal Officer | | | 2016 | | | $ | 693,663 | | | | — | | | $ | 2,337,051 | | | | $ 589,514 | | | | $133,726 | | | | $171,114 | | | $ | 3,925,068 | | | 2018 | | | $ | 746,254 | | | | — | | | $ | 4,359,590 | | | $ 858,744 | | | $ 188,056 | | | $ 201,140 | | | $ | 6,353,784 | | and Secretary | | | Paul F. McKenzie | | | 2018 | | | $ | 630,455 | | | | — | | | $ | 4,083,884 | | | | $ 784,784 | | | | $ 12,367 | | | | $154,406 | | | $ | 5,665,896 | | | And Secretary | | | | | | | | | | | | | | | | | | Chirfi Guindo | | | | 2020 | | | $ | 562,819 | | | | — | | | $ | 3,961,285 | | | | $ 527,970 | | | | — | | | | $ 80,721 | | | $ | 5,132,795 | | Executive Vice President, | | | 2017 | | | $ | 600,433 | | | | — | | | $ | 3,514,451 | | | | $ 796,648 | | | | $ 2,471 | | | | $101,254 | | | $ | 5,015,257 | | | 2019 | | | $ | 526,539 | | | | — | | | $ | 3,379,426 | | | $ 706,384 | | | | — | | | $ 82,173 | | | $ | 4,694,522 | | Pharmaceutical Operations & Technology | | | | Global Product Strategy and Commercialization | | | | | | | | | | | | | | | | | | Jeffrey D. Capello(7) | | | | 2020 | | | $ | 671,132 | | | | — | | | $ | 3,901,503 | | | | — | | | | — | | | | $4,648,876 | | | $ | 9,221,511 | | Former Executive Vice | | | 2019 | | | $ | 783,173 | | | | — | | | $ | 4,051,891 | | | $ 883,575 | | | | — | | | $ 95,450 | | | $ | 5,814,089 | | President and Chief | | | 2018 | | | $ | 750,000 | | | | — | | | $ | 2,889,224 | | | $ 790,913 | | | | — | | | $ 46,582 | | | $ | 4,476,719 | | Financial Officer | | | | | | | | | | | | | | | | | |
Notes to the Summary Compensation Table | (1) | The amountsamount in column (d) reflectreflects a sign-on bonusesbonus paid to Messrs. Vounatsos and Capello and Dr. Ehlers at the time ofMr. McDonnell in connection with his hire. All other cash bonuses, which were based on achievement of performance goals under our annual bonus plan, are disclosed in column (f). |
| (2) | The amounts in column (e) reflect the grant date fair value, computed in accordance with ASC 718, for RSUs, MSUs PSUs and CSPUsPSUs granted during 2018, 20172020, 2019 and 2016,2018, as applicable, excluding the effect of estimated forfeitures. The cash portion of PSUs are included in the year when the applicable performance goals are set and the fair value of the PSUs is determinable. The 2020 amounts include one-third of the 2020 Cash-Settled PSUs, one-third of the 2019 Cash-Settled PSUs and one-third of the 2018 Cash-Settled PSUs, which are the tranches of the awards for which performance goals were set in 2020 relating to the 2020 performance period. The 2020 amounts also include a one-time hire award of RSUs granted to Mr. McDonnell as described under the heading “2020 Hiring-Related Compensation Decisions—Arrangements with Mr. McDonnell.” The 2019 amounts include one-third of the 2019 Cash-Settled PSUs and one-third of the 2018 Cash-Settled PSUs, which are the tranches of the awards for which performance goals were set in 2019 relating to the 2019 performance period. The 2018 amounts includeone-third of the 2018 Cash-Settled PSUs, which is the tranche of the award for which performance goals had beenwere set in 2018 relating to the 2018 performance period. The 2018 amounts also includeone-time transition awards of RSUs granted to each of the NEOs, except Messrs. VounatsosDr. Sandrock and Capello, as described under “2018One-Time Transition Awards” above. The 2017 amounts for Dr. McKenzie represent grants of MSUs, CSPUs and RSUs, as described in “Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie” in our 2018 proxy statement. The 2016 amounts for Dr. Ehlers represent grants of MSUs, CSPUs and RSUs as described in “Executive Compensation Matters—Compensation Discussion and Analysis—2016 and 2017 Hiring- and Transition-Related Compensation Decisions—Arrangements with Mr. Vounatsos and Dr. Ehlers” in our 2017 proxy statement. The awards granted before February 1, 2017, were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts reported in this column were not impacted by such anti-dilution adjustments.Ms. Alexander. The grant date fair value for MSU awards are estimated as of the date of grant using a lattice model with a Monte Carlo simulation, based on the probable outcome of applicable performance conditions. The grant date fair value for PSU CSPU and RSU awards was determined by multiplying the number of shares subject to the award (assuming target performance for PSUs and CSPUs)such PSUs) by the closing price of the Company’s common stock on the grant date. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 1615 of our 20182020 Annual Report on Form10-K. The table below shows the target and maximum payouts possible for the 2020, 2019 and 2018 2017MSU and 2016 MSU, PSU and CSPU awards based on the grant date fair value at the date of grant and theassuming target and maximum payout levels. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2018 | | | 2017 | | | 2016 | | | | | | | | | | Executive Officer | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | Mr. Vounatsos | | | $11,064,897 | | | | $22,129,794 | | | | $9,868,540 | | | | $19,737,080 | | | | $3,151,199 | | | | $6,302,398 | | Mr. Capello | | | $ 2,889,224 | | | | $ 5,778,448 | | | | — | | | | — | | | | — | | | | — | | Dr. Ehlers | | | $ 3,608,292 | | | | $ 7,216,584 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,540,438 | | | | $5,080,876 | | Ms. Alexander | | | $ 3,078,822 | | | | $ 6,157,644 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,337,051 | | | | $4,674,102 | | Dr. McKenzie | | | $ 2,883,856 | | | | $ 5,767,712 | | | | $2,713,851 | | | | $ 5,427,702 | | | | — | | | | — | |
| | | | | | | 52 | | 58 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2020 | | | 2019 | | | 2018 | | | | | | | | | | Executive Officer | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | Mr. Vounatsos | | | $13,887,064 | | | | $27,774,128 | | | | $12,352,030 | | | | $24,704,060 | | | | $11,064,897 | | | | $22,129,794 | | Mr. McDonnell | | | $ 2,761,719 | | | | $ 5,523,437 | | | | — | | | | — | | | | — | | | | — | | Dr. Sandrock | | | $ 4,331,654 | | | | $ 8,663,308 | | | | $ 3,360,682 | | | | $ 6,721,364 | | | | — | | | | — | | Ms. Alexander | | | $ 3,583,281 | | | | $ 7,166,562 | | | | $ 3,302,619 | | | | $ 6,605,238 | | | | $ 3,078,822 | | | | $ 6,157,644 | | Mr. Guindo | | | $ 3,961,285 | | | | $ 7,992,570 | | | | $ 3,379,426 | | | | $ 6,758,852 | | | | — | | | | — | | Mr. Capello | | | $ 3,901,503 | | | | $ 7,803,006 | | | | $ 4,051,891 | | | | $ 8,103,782 | | | | $ 2,889,224 | | | | $ 5,778,448 | |
| (3) | The amounts in column (f) reflect actual bonuses paid under our annual bonus plan for the applicable year. |
| (4) | The amounts in column (g) reflect earnings in the SSP that are in excess of 120% of the applicable federal long-term rate. The federal long-term rates applied in this calculation are 3.06%1.61%, 3.26%3.73% and 3.14%3.06% for 2018, 20172020, 2019 and 2016,2018, respectively. The SSP is described under the heading “2018“2020 Non-Qualified Deferred Compensation” below. |
| (5) | The amounts in column (h) for 20182020 reflect the following: |
| | | Executive Officer | | Company Matching Contribution to 401(k) Plan Account | | Company Contribution to SSP Account | | Personal Health and Financial Planning(8) | | Value of Company- Paid Life Insurance Premiums | | Relocation(9) | | Company Matching Contribution to 401(k) Plan Account | | | Company Contribution to SSP Account | | | Personal Health and Financial Planning(8) | | | Value of Company- Paid Life Insurance Premiums | | | Severance(9) | | Mr. Vounatsos | | $ | 16,500 | | | $ | 387,134 | | | $ | 3,594 | | | $ | 1,055 | | | | — | | | $ | 17,100 | | | $ | 433,320 | | | $ | 3,470 | | | $ | 1,055 | | | | — | | Mr. McDonnell | | | | — | | | | — | | | $ | 5,037 | | | $ | 659 | | | | — | | Dr. Sandrock | | | $ | 17,100 | | | $ | 120,725 | | | $ | 7,500 | | | $ | 1,695 | | | | — | | Ms. Alexander | | | $ | 17,100 | | | $ | 134,365 | | | $ | 6,160 | | | $ | 1,636 | | | | — | | Mr. Guindo | | | $ | 17,100 | | | $ | 58,453 | | | $ | 4,050 | | | $ | 1,118 | | | | — | | Mr. Capello | | $ | 16,500 | | | $ | 28,500 | | | | — | | | $ | 1,582 | | | | — | | | $ | 17,100 | | | $ | 72,806 | | | $ | 3,540 | | | $ | 1,662 | | | $ | 4,553,768 | | Dr. Ehlers | | $ | 16,500 | | | $ | 168,428 | | | | — | | | $ | 1,582 | | | | — | | | Ms. Alexander | | $ | 16,500 | | | $ | 176,553 | | | $ | 6,560 | | | $ | 1,527 | | | | — | | | Dr. McKenzie | | $ | 16,500 | | | $ | 118,612 | | | $ | 5,179 | | | $ | 1,274 | | | $ | 12,841 | | |
| (6) | Mr. Vounatsos joined Biogen as our Executive Vice President, Chief Commercial Officer effective April 18, 2016. Mr. Vounatsos was appointed our Chief Executive Officer and a member of our Board of Directors effective January 6, 2017. His base salary for 2017 was $1,100,000, of which he received a pro rata share from January 6, 2017 to December 31, 2017. From January 1, 2017 to January 5, 2017, he was paid at his prior rate of base salary ($750,000). |
(7) | Mr. CapelloMcDonnell was appointed as our Executive Vice President and Chief Financial Officer effective December 11, 2017.August 15, 2020. His base salary and annual bonus for 2017 was $750,000,2020 were prorated for the period of the year during which he received a pro rata sharewas employed by the Company.
|
| (7) | Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer on August 15, 2020, and separated from December 11, 2017 to December 31, 2017.the Company on September 15, 2020. Mr. Capello’s base salary was prorated for the period of the year during which he was employed by the Company. Mr. Capello was not eligible to participate inreceive a bonus under our 20172020 annual bonus plan and, was not granted any stock awards in 2017.connection with his termination of employment, all of his then unvested RSUs, MSUs and PSUs were forfeited. |
| (8) | Represents reimbursements of expenses relating to tax, financial and estate planning and executive physicals as described under the heading “Executive Physicals, Tax Preparation, Financial and Estate Planning” above. |
| (9) | Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer on August 15, 2020, and separated from the Company on September 15, 2020. The amountCompany provided him the severance benefits required under our executive severance policy for Dr. McKenzie reflects relocationExecutive Vice Presidents, which consisted of (a) a lump sum cash payment of $1,892,625 (16 months of base salary and target bonus), (b) continuation of certain subsidized medical, dental and vision benefits underuntil the Company’s Executive Relocation Policyearlier of (1) December 31, 2021, or (2) the date on which he becomes eligible to receive benefits through another employer and includes(c) up to 12 months of executive-level outplacement services at a taxcost of up to $32,000. In addition, in recognition of Mr. Capello’s contributions to the Company and to facilitate a successful transition to Mr. McDonnell, our C&MD Committee waived the requirement that Mr. Capello repay 35% of his cash gross-upsign-on bonus and approved an additional cash payment to him of $1,518.$2,600,000. The cost of the continuation of certain subsidized medical, dental and vision benefits equaled (assuming the benefits continue until December 31, 2021) is $29,143 and also included the employer portion of premiums for the period April 1, 2021 through September 30, 2021, pursuant to the American Rescue Plan Act of 2021. |
| | | | | | | 53 | | 59 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
20182020 Grants of Plan-Based Awards
The following table shows additional information regarding all grants of plan-based awards made to our NEOs for the year ended December 31, 2018.2020. | | | | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan
Awards(1) | | | | Estimated Future Payouts
Under Equity Incentive Plan
Awards(#)(1) | | All Other Stock Awards: Number of
Shares or Units
(#) (i) | | Grant Date Fair Value of Stock Awards(2) (j) | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan
Awards(1) | | | | | | Estimated Future Payouts
Under Equity Incentive Plan
Awards(#)(1) | | All Other Stock Awards: Number of
Shares or Units
(#) (i) | | Grant Date Fair Value of Stock Awards(2) (j) | | Name (a) | | Grant Date (b) | | Notes | | Threshold (c) | | Target (d) | | Maximum (e) | | | | Threshold (f) | | Target (g) | | Maximum (h) | | Grant Date (b) | | Notes | | Threshold (c) | | Target (d) | | Maximum (e) | | | | Threshold (f) | | Target (g) | | Maximum (h) | Michel Vounatsos | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 9,080 | | | | 18,160 | | | | 36,320 | | | | — | | | $ | 6,856,251 | | | | 02/12/2020 | | | (3) | | | — | | | | — | | | | — | | | | | 9,420 | | 18,840 | | 37,680 | | — | | $ | 7,669,882 | | | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 6,646 | | | | 13,292 | | | | 26,584 | | | | — | | | $ | 4,208,646 | | | | 02/12/2020 | | | (4) | | | — | | | | — | | | | — | | | | | 9,370 | | 18,740 | | 37,480 | | — | | $ | 6,217,182 | | | | | | 02/12/2018 | | | (5) | | $ | 455,000 | | | $ | 1,820,000 | | | $ | 4,095,000 | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 02/12/2020 | | | (5) | | $ | 562,500 | | | $ | 2,250,000 | | | $ | 5,062,500 | | | | | — | | — | | — | | — | | | — | | Jeffrey D. Capello | | | 01/02/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,245 | | | | 4,490 | | | | 8,980 | | | | — | | | $ | 1,789,136 | | | Michael R. McDonnell | | | | 09/01/2020 | | | (3) | | | — | | | | — | | | | �� | | | | | 4,023 | | 8,045 | | 16,090 | | — | | $ | 2,761,719 | | | | | 01/02/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,646 | | | | 3,292 | | | | 6,584 | | | | — | | | $ | 1,100,088 | | | | 09/01/2020 | | | (5) | | | $ 60,257 | | | | $ 241,027 | | | | $ 542,312 | | | | | — | | — | | — | | — | | | — | | | | | | 02/12/2018 | | | (5) | | $ | 131,250 | | | $ | 525,000 | | | $ | 1,181,250 | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 09/01/2020 | | | (6) | | | — | | | | — | | | | — | | | | | — | | — | | — | | 8,045 | | | $2,250,589 | | Michael D. Ehlers | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,960 | | | | 5,920 | | | | 11,840 | | | | — | | | $ | 2,235,068 | | | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 2,169 | | | | 4,337 | | | | 8,674 | | | | — | | | $ | 1,373,224 | | | Alfred W. Sandrock, Jr. | | | | 02/12/2020 | | | (3) | | | — | | | | — | | | | — | | | | | 3,015 | | 6,030 | | 12,060 | | — | | $ | 2,454,888 | | | | | 02/12/2018 | | | (5) | | $ | 145,966 | | | $ | 583,866 | | | $ | 1,313,698 | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 02/12/2020 | | | (4) | | | — | | | | — | | | | — | | | | | 2,829 | | 5,657 | | 11,314 | | — | | $ | 1,876,766 | | | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 4,735 | | | $ | 1,499,243 | | | | 02/12/2020 | | | (5) | | $ | 169,065 | | | $ | 676,260 | | | $ | 1,521,585 | | | | | — | | — | | — | | — | | | — | | Susan H. Alexander | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,528 | | | | 5,055 | | | | 10,110 | | | | — | | | $ | 1,908,558 | | | | 02/12/2020 | | | (3) | | | — | | | | — | | | | — | | | | | 2,413 | | 4,825 | | 9,650 | | — | | $ | 1,964,292 | | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,848 | | | | 3,696 | | | | 7,392 | | | | — | | | $ | 1,170,264 | | | | 02/12/2020 | | | (4) | | | — | | | | — | | | | — | | | | | 2,440 | | 4,880 | | 9,760 | | — | | $ | 1,618,989 | | | | | 02/12/2018 | | | (5) | | $ | 131,106 | | | $ | 524,424 | | | $ | 1,179,954 | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 02/12/2020 | | | (5) | | $ | 142,479 | | | $ | 569,918 | | | $ | 1,282,315 | | | | | — | | — | | — | | — | | | — | | | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 4,045 | | | $ | 1,280,768 | | | Paul F. McKenzie | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,368 | | | | 4,735 | | | | 9,470 | | | | — | | | $ | 1,787,683 | | | Chirfi Guindo | | | | 02/12/2020 | | | (3) | | | — | | | | — | | | | — | | | | | 2,863 | | 5,725 | | 11,450 | | — | | $ | 2,330,685 | | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,731 | | | | 3,462 | | | | 6,924 | | | | — | | | $ | 1,096,173 | | | | 02/12/2020 | | | (4) | | | — | | | | — | | | | — | | | | | 2,458 | | 4,915 | | 9,830 | | — | | $ | 1,630,600 | | | | | 02/12/2018 | | | (5) | | $ | 110,939 | | | $ | 443,757 | | | $ | 998,452 | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 02/12/2020 | | | (5) | | $ | 99,243 | | | $ | 396,970 | | | $ | 893,183 | | | | | — | | — | | — | | — | | | — | | Jeffrey D. Capello(7) | | | | 02/12/2020 | | | (3) | | | — | | | | — | | | | — | | | | | 2,638 | | 5,275 | | 10,550 | | — | | $ | 2,147,488 | | | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 3,790 | | | $ | 1,200,028 | | | | 02/12/2020 | | | (4) | | | — | | | | — | | | | — | | | | | 2,644 | | 5,287 | | 10,574 | | — | | $ | 1,754,015 | | | | | | | 02/12/2020 | | | (5) | | $ | 152,086 | | | $ | 608,344 | | | $ | 1,368,773 | | | | | — | | — | | — | | — | | | — | |
Notes to the 20182020 Grants of Plan-Based Awards Table | (1) | Reflects the potential future payouts of awards granted in 20182020 under our 20182020 annual bonus plan and our LTI program for each NEO as of the respective grant dates. |
| (2) | Represents the grant date fair value of PSUs, MSUs and RSUs, as applicable, computed in accordance with ASC 718, excluding the effect of estimated forfeitures. The grant date fair value for MSU awards is estimated as of the date of grant using a lattice model with a Monte Carlo simulation based on the probable outcome of applicable performance conditions. TheIn addition to the grant date fair value of the 2020 Stock-Settled PSUs, for all NEOs other than Mr. McDonnell, the grant date fair value for both PSU and RSU awards is determined by multiplying the number of shares subject to the award (assuming target performance for PSUs)performance) by the closing price of our common stock on the grant date. ForThe grant date value of one-third of the 2020 Cash-Settled PSUs, one third of the 2019 Cash-Settled PSUs and one-third of the 2018 Cash-Settled PSUs are included in 2020, which are the grant date value ofone-thirdtranches of the award is included, which is the tranche of the awardawards for which performance goals had beenwere set relating to the 20182020 performance period and the fair value was determinable in 2018.2020. The grant date fair value of the remaining tranches of the 20182019 and 2020 Cash-Settled PSUs will be included, as applicable, in the compensation tables for 20192021 and 2020,2022, the years when performance goals will be set with respect to such performance periods and fair value iswill be determinable. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 1615 of our 20182020 Annual Report on Form10-K. The maximum payouts for these awards are included in the footnotes following the Summary Compensation Table above. |
| (3) | These amounts relate to the annual grant of MSUs. MSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). These are performance-based RSUs tied to the growth in our stock price between the grant date and each of three annual vesting dates. The number of MSUs earned is determined on each vesting date. Columns (f), (g) and (h) represent the number of MSUs that can be earned based on performance at the threshold level of 50%, target level of 100% and the maximum level of 200%, respectively. To the extent earned, the award becomes eligible to vest ratably over three years, generally subject to continued service, as described in further detail under the heading “Long-Term Incentives” above. |
| (4) | These amounts relate to the annual grant of PSUs. PSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). The PSUs have three-year cliff vesting. The amounts shown include the 20182020 Stock-Settled PSUs, andone-third of the 2018 Cash-Settled PSUs, which is the trancheone-third of the award2019 Cash-Settled PSUs and one-third of the 2020 Cash-Settled PSUs, which are the tranches of the awards for which performance goals had beenwere set in 20182020 relating to the 20182020 performance period and the fair value was determinable in 2018.2020. The remaining tranches of the 20182019 and 2020 Cash-Settled PSUs will be included, as applicable, in the compensation tables for 20192021 and 2020,2022, the years when performance goals will be set with respect to such performance periods and fair value iswill be determinable. Columns (f), (g) and (h) represent the number of 20182020 Stock-Settled PSUs and the 20182020 tranche of the 2018, 2019 and 2020 Cash-Settled PSUs that can be earned if the Company Multiplier were 50%, 100% and 200%, respectively. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs”Incentives” above. |
| | | | | | | | | 60 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| (5) | These amounts relate to our 20182020 annual bonus plan. The amounts shown in column (d) represent the 20182020 target payout amount based on the target percentage applied to each NEO’s base salary as of December 31, 2018.2020. For 20182020 the bonus targets were 140%150% of base salary for Mr. Vounatsos, 75% of base salary for Messrs. McDonnell and Capello and Dr. Sandrock and 70% of base salary for all other NEOs.Ms. Alexander and Mr. Guindo. For Mr. McDonnell, the amounts reported are based on a prorated bonus target for 2020 based on his August 15, 2020, hire date, as described in further detail under the heading “Annual Bonus Plan” above. The amounts in column (c), (d) and (e) represent a payment if the Company Multiplier and the Individual Multiplier were each 50%, 100% and 150%, respectively. Actual amounts paid to each NEO |
| | | | | 54 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
| under our 20182020 annual bonus plan are included in the“Non-Equity “Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table above. |
| (6) | These amounts relate to a one-time transition awards grant of time-based RSUs granted to each of the NEOs, except Messrs. Vounatsos and Capello,for Mr. McDonnell, as described in further detail in the CD&A above under “2018One-Time Transition Awards” above.the heading “2020 Hiring-Related Compensation Decisions—Arrangements with Mr. McDonnell.” |
| (7) | Mr. Capello separated from the Company on September 15, 2020. As a result of his termination of employment, Mr. Capello forfeited all then unvested RSUs, MSUs and PSUs that were held by him and was not eligible to receive a bonus for 2020 under our 2020 annual bonus plan. |
Outstanding Equity Awards at 20182020 FiscalYear-End The following table summarizes the equity awards that were outstanding as of December 31, 2018,2020, for each of our NEOs. | | | | | | | Option Awards | | Stock Awards | | | | | | | | Stock Awards | | | | | | | | | | | | | | | | | | | | Equity Incentive Plan
Awards | | | | | | | | | | | | | | Equity Incentive Plan
Awards | | | | | Grant
Date (b) | | Notes | | Number of Securities Underlying Unexercised
Options | | | Option Exercise Price (e) | | Option Expiration Date (f) | | Number of Shares or Units of Stock That Have Not Vested (g) | | Market Value of Shares or Units of Stock That Have Not Vested (h) | | Number of Unearned Shares or Units That Have Not Vested (i) | | Market Value of Unearned Shares or Units That Have Not Vested(1) (j) | | | Grant
Date (b) | | | Notes | | Number
of
Shares
or Units
of Stock
That
Have
Not
Vested (c) | | | Market
Value of
Shares or
Units of
Stock That Have
Not Vested (d) | | | Number
of
Unearned
Shares or
Units
That
Have Not
Vested (e) | | | Market
Value of
Unearned
Shares or
Units That
Have Not
Vested(1) (f) | | | (a) | | Exercisable (c) | | Unexercisable (d) | | | Michel Vounatsos | | | 5/2/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 2,320 | | | $ | 698,134 | | | | — | | | | — | | | | 2/12/2018 | | | (2) | | | — | | | | — | | | | 6,056 | | | $ | 1,482,872 | | | | | 5/2/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,252 | | | $ | 978,592 | | | | 2/12/2018 | | | (3) | | | 27,028 | | | $ | 6,618,076 | | | | — | | | | — | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 14,080 | | | $ | 4,236,954 | | | | — | | | | — | | | | 2/12/2019 | | | (2) | | | — | | | | — | | | | 12,524 | | | $ | 3,066,627 | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 17,002 | | | $ | 5,116,242 | | | | 2/12/2019 | | | (3) | | | 6,222 | | | $ | 1,523,519 | | | | 13,827 | | | $ | 3,385,679 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 36,320 | | | $ | 10,929,414 | | | | 2/12/2020 | | | (2) | | | — | | | | — | | | | 18,840 | | | $ | 4,613,162 | | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 4,602 | | | $ | 1,384,834 | | | | 15,763 | | | $ | 4,743,402 | | | | 2/12/2020 | | | (3) | | | 2,237 | | | $ | 547,752 | | | | 16,355 | | | $ | 4,004,685 | | Jeffrey D. Capello | | | 1/2/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,490 | | | $ | 1,351,131 | | | Michael R. McDonnell | | | | 9/1/2020 | | | (2) | | | — | | | | — | | | | 8,045 | | | $ | 1,969,899 | | | | | | 1/2/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,146 | | | $ | 344,854 | | | | 3,893 | | | $ | 1,171,482 | | | | 9/1/2020 | | | (4) | | | 8,045 | | | $ | 1,969,889 | | | | — | | | | — | | Michael D. Ehlers | | | 6/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,821 | | | $ | 547,975 | | | | — | | | | — | | | | | | 6/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,502 | | | $ | 752,902 | | | | | | 6/1/2016 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,036 | | | $ | 311,753 | | | | — | | | | — | | | Alfred W. Sandrock, Jr. | | | | 2/12/2018 | | | (2) | | | — | | | | — | | | | 1,383 | | | $ | 338,641 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | | | | 2/12/2018 | | | (3) | | | 6,168 | | | $ | 1,510,296 | | | | — | | | | — | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | | | | 2/12/2019 | | | (2) | | | — | | | | — | | | | 3,444 | | | $ | 843,298 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 11,840 | | | $ | 3,562,893 | | | | 2/12/2019 | | | (3) | | | 1,709 | | | $ | 418,466 | | | | 3,803 | | | $ | 931,203 | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,501 | | | $ | 451,681 | | | | 5,143 | | | $ | 1,547,632 | | | | 2/12/2020 | | | (2) | | | — | | | | — | | | | 6,030 | | | $ | 1,476,506 | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,735 | | | $ | 1,424,856 | | | | — | | | | — | | | | 2/12/2020 | | | (3) | | | 716 | | | $ | 175,320 | | | | 5,230 | | | $ | 1,280,618 | | Susan H. Alexander | | | 2/24/2009 | | | (6) | | | 6,395 | | | | — | | | | $48.52 | | | | 2/23/2019 | | | | — | | | | — | | | | — | | | | — | | | | 2/12/2018 | | | (2) | | | — | | | | — | | | | 1,687 | | | $ | 413,079 | | | | | 2/22/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,806 | | | $ | 543,462 | | | | — | | | | — | | | | 2/12/2018 | | | (3) | | | 7,515 | | | $ | 1,840,123 | | | | — | | | | — | | | | | 2/22/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,482 | | | $ | 746,883 | | | | 2/12/2019 | | | (2) | | | — | | | | — | | | | 3,341 | | | $ | 818,077 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | | | | 2/12/2019 | | | (3) | | | 1,659 | | | $ | 406,223 | | | | 3,688 | | | $ | 903,044 | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | | | | 2/12/2020 | | | (2) | | | — | | | | — | | | | 4,825 | | | $ | 1,181,450 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,110 | | | $ | 3,042,301 | | | | 2/12/2020 | | | (3) | | | 572 | | | $ | 140,060 | | | | 4,189 | | | $ | 1,025,719 | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,279 | | | $ | 384,877 | | | | 4,384 | | | $ | 1,319,233 | | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,045 | | | $ | 1,217,221 | | | | — | | | | — | | | Paul F. McKenzie | | | 3/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 616 | | | $ | 185,367 | | | | — | | | | — | | | Chirfi Guindo | | | | 2/12/2019 | | | (2) | | | — | | | | — | | | | 3,654 | | | $ | 894,718 | | | | | 3/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 848 | | | $ | 255,180 | | | | 2/12/2019 | | | (3) | | | 1,817 | | | $ | 444,911 | | | | 4,032 | | | $ | 987,276 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 3,875 | | | $ | 1,166,065 | | | | — | | | | — | | | | 2/12/2020 | | | (2) | | | — | | | | — | | | | 5,725 | | | $ | 1,401,824 | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,674 | | | $ | 1,406,500 | | | | 2/12/2020 | | | (3) | | | 680 | | | $ | 166,505 | | | | 4,974 | | | $ | 1,217,934 | | | | | 3/1/2017 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,820 | | | $ | 547,674 | | | | — | | | | — | | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,470 | | | $ | 2,849,712 | | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,194 | | | $ | 359,298 | | | | 4,108 | | | $ | 1,236,179 | | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 3,790 | | | $ | 1,140,487 | | | | — | | | | — | | | Jeffrey D. Capello | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Notes to the Outstanding Equity Awards at 20182020 Fiscal Year End Table | (1) | The market value of awards is based on the closing price of our stock on December 31, 20182020 ($300.92)244.86), as reported byon Nasdaq. |
| (2) | CSPUs were granted in 2017 and 2016. The numbers in column (g) reflect the number of CSPUs that were earned based on our financial performance for each of 2017 and 2016, but that had not vested based on our service-based vesting requirement as of December 31, 2018. CSPUs that have been earned based on the satisfaction of the performance conditions vest ratably over three years from the grant date. The cash payout for these awards will be based on our30-day average closing stock price at vesting. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts.
|
(3) | MSUs were granted in 2018, 20172020, 2019 and 2016.2018. These are performance-based RSUs earned based on the growth in our stock price between the dates of grant and vesting. Earned MSUs are eligible to vest ratably overin equal annual installments on each of the first three years.anniversaries of the grant date, generally subject to continued employment through the applicable vesting date. The number and value shown in columns (i)(e) and (j)(f), respectively, reflects maximumreflect target performance results for MSUs granted in 2018, 2017 and 2016 based on the estimated performance atyear-end in each case except for Mr. Capello’s January 2, 2018, grant, for which columns (i) and (j) reflect target performance results based on the estimated performance atyear-end. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts.case. |
| | | | | 55 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
(4)(3) | PSUs were granted in 2020, 2019 and 2018. The PSUs, areto the extent earned, cliff vest on the third anniversary of the date of grant, generally subject to three-year cliff vesting. In addition,continued employment through the vesting date. 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock and performance will beis based upon achievement of cumulative three-year pipeline goals for the 2020 award and cumulative three-year financial and pipeline goals.goals for the 2019 and 2018 awards. The remaining 40% of the PSUs will |
| | | | | | | | | 61 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| be settled in cash and performance will beis based upon the achievement of three annual financial goals determined at the beginning of each relevant year. The number and value shown in columns (g)(c) and (h)(d), respectively, reflect the number of 2020 Cash-Settled PSUs, 2019 Cash-Settled PSUs and 2018 Cash-Settled and Stock-Settled PSUs that were earned based on our achievement of the annual performance goals, for the 2018 tranche, but that will not vest until February 12, 2021.2023, February 12, 2022, and February 12, 2021, respectively. The number and value shown in columns (i)(e) and (j)(f), respectively, reflect the remaining portion of the PSUs granted in 20182020 and 2019 (including the 20192021 and 20202022 tranches of the 20182020 Cash-Settled PSUs and the 2021 tranche of the 2019 Cash-Settled PSUs) assuming target performance results. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs”Incentive” above. |
(5)(4) | RSUs were granted in 2018, 2017 and 2016 for special circumstances. 2018 RSU awards relate toone-time transition awards granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described under “2018One-Time Transition Awards” above. For Dr. Ehlers and Dr. McKenzie, the amounts in this column alsofor Mr. McDonnell reflect 3,104 RSUs granted to Dr. Ehlers on JuneSeptember 1, 2016,2020, in connection with his hiring and 2,730 RSUs granted to Dr. McKenzie on March 1, 2017, under hisone-time award, as described in more detail under the heading “Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie” in our 2018 proxy statement, in each case vesting ratably over three years from the grant date. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts.
|
(6) | These stock options were granted with aten-year term. Stock options vest 25% on each of the first four anniversaries of the grant date. These stock options were adjusted pursuant to the anti-dilution provisions of the award in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share and exercise price amounts.hiring.
|
20182020 Option Exercises and Stock Vested
Our executive officers must usepre-established trading plans to sell shares of our common stock. Trading plans may only be entered into during an open trading window and when the executive is not in possession of materialnon-public information about the Company, and we require a waiting period following the establishment of a trading plan before any trades may be executed. Our policy is designed to provide safeguards while allowing our executives an opportunity to realize the value intended by the Company in granting equity-based LTI awards. Our NEOs are also subject to the stock ownership guidelines described above under the heading “Stock Ownership Guidelines.” The following table shows information regarding the exercise of stock options and the vesting of stock awards for each NEO during the year ended December 31, 2018. None of the NEOs exercised stock options during the year ended December 31, 2018.2020. | | | | | Stock Awards | | Stock Awards | | Name | | Number of Shares
Acquired on
Vesting(1) | | | | Value
Realized on
Vesting(2)(3) | | Number of Shares
Acquired on
Vesting(1) | | | | Value
Realized on
Vesting(2) | Michel Vounatsos | | | 16,294 | | | $ | 5,013,149 | | | | | 23,986 | | | | | | | $ | 7,754,636 | | Michael R. McDonnell | | | | | — | | | | | | | | — | | Alfred W. Sandrock, Jr. | | | | | 8,215 | | | | | | | $ | 2,674,660 | | Susan H. Alexander | | | | | 9,807 | | | | | | | $ | 3,188,471 | | Chirfi Guindo | | | | | 2,945 | | | | | | | $ | 864,954 | | Jeffrey D. Capello | | | — | | | | — | | | | | 3,221 | | | | | | | $ | 1,019,748 | | Michael D. Ehlers | | | 8,115 | | | $ | 2,471,489 | | | Susan H. Alexander | | | 9,111 | | | $ | 2,844,100 | | | Paul F. McKenzie | | | 5,419 | | | $ | 1,672,605 | | |
Notes to the 20182020 Option Exercises and Stock Vested Table | (1) | CSPUsCash-settled performance units (CSPUs) that were granted in 2017 were settled in cash for all of our NEOs.Mr. Vounatsos, Dr. Sandrock and Ms. Alexander. The number of actual shares of our common stock acquired on vesting with respect toof MSUs and RSUs as applicable,in 2020, after shares were withheld to pay the minimum withholding of taxes, was as follows:
|
| | | | | | | | | | | Net Shares Acquired(4)(3) | Mr. Vounatsos | | | 4,414
| 9,796 | | Mr. McDonnell | | | | — | | Dr. Sandrock | | | | 3,990 | | Ms. Alexander | | | | 4,596 | | Mr. Guindo | | | | 1,879 | | Mr. Capello | | —
| Dr. Ehlers
| | 2,545
| Ms. Alexander
| | 2,586
| Dr. McKenzie
| | 1,830
|
| | | | | 56 | |  | |  |
| | | 5 | | | Executive Compensation Matters (continued)2,201
| |
| (2) | The value realized for MSUs and RSUs arewas calculated by multiplying the closing price of a share of our common stock on the vesting date by the total number of shares that vested on such date. The value realized for CSPUs is calculated using the30-day average closing price of the common stock of the Company through the vesting date. |
| (3) | The value realized upon vesting for Dr. McKenzie includes CSPUs with a value of $304,487, the receipt of which was deferred under the SSP, as described below. Terms of thenon-qualified deferred compensation plan are described under the heading “2018Non-Qualified Deferred Compensation” below.
|
(4) | MSUs and RSUs were settled in shares of our common stock. CSPUs were settled in cash. |
| | | | | | | | | 62 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
20182020 Non-Qualified Deferred Compensation
The SSP covers our executive officers and other eligible employees in the U.S. Employees whose base salary and annual cash incentives for the year exceed a specified Internal Revenue ServicesService’s limit ($275,000285,000 in 2018)2020) receive a Company-paid restoration match on the portion of their base salary, annual bonus and cash payments in respect of CSPUs and cash-settled PSUs, as applicable, that exceeds this limit; the restoration match equals 6% of this excess compensation. The restoration match feature is intended to provide the amount of matching employer contributions that the participant would otherwise have been eligible to receive under our 401(k) plan but for the $275,000$285,000 limit imposed by Section 401(a)(17) of the Internal Revenue Code. In addition, eligible employees may make voluntary contributions of up to 80% of their base salary and 100% of their annual bonus and cash payments in respect of CSPUs and cash-settled PSUs, as applicable, to the SSP, and thereby defer income taxes on such amounts until distribution is made from the SSP. The Company does not match participants’ voluntary contributions to the SSP. The SSP provides for immediate vesting of the restoration match consistent with our immediate vesting of the Company match provided under our 401(k) plan. Notional SSP accounts are maintained for each participant. Accounts include employee and employer contributions and reflect the performance of notional investments selected by the employee or a default investment if the employee does not make a selection. These notional investment options include the mutual funds offered under our 401(k) plan as well as a fixed rate option which earns a rate of return determined each year by the Company’s retirement committee. For contributions to the SSP fixed rate option in 2018,2020, this rate of return was set at 5%. Contributions to the fixed rate option continue to earn interest at the rate of return that was in effect during the year of contribution. The excess of the interest rate paid on the fixed rate option above 120% of the applicable federal long-term rate (compounded quarterly) earned by our NEOs during 20182020 is shown in the Summary Compensation Table. We fund the SSP liabilities through corporate-owned life insurance (COLI), which we purchase with the written consent of SSP participants, and investments in mutual funds. We believe that the COLI policies and mutual funds will be sufficient to cover plan liabilities through the projected payout date so the plan will not require direct funding by the Company. Upon enrollment in the SSP, a participant must elect when and how distributions will be made from the participant’s account. Distributions can be made upon termination of the participant’s employment, either in a lump sum or up to 15 annual installments, or at a specified future date while the participant is still employed (an“in-service” “in-service” distribution), either in a lump sum or up to 5 annual installments. Further, upon enrollment, a participant must also elect a distribution method upon death or a change in control of the Company, which can either be a lump sum payment or, if different, the method selected for payment upon termination of employment. The following table shows a summary of all contributions to, earnings on and distributions received from the SSP for each of our NEOs for the year ended December 31, 2018.2020. The account balances as ofyear-end include all contributions and interest amounts earned by our NEOs through the end of 20182020 plus the SSP contributions that the Company made in early 20192021 based on earnings in the last quarter of 2018.2020. | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Executive
Contributions
in Last Fiscal
Year(1) | | | Company
Contributions
in Last Fiscal
Year(2) | | | Aggregate
Earnings
in Last
Fiscal
Year(3) | | | Aggregate
Distributions
in Last
Fiscal Year | | Aggregate
Balance at
Last Fiscal Year-End(4) | | Michel Vounatsos | | $ | 3,071,852 | | | $ | 387,134 | | | $ | 205,737 | | | — | | $ | 5,160,044 | | Jeffrey D. Capello | | | — | | | $ | 28,500 | | | $ | 60 | | | — | | $ | 28,560 | | Michael D. Ehlers | | $ | 552,463 | | | $ | 168,428 | | | $ | (80,992 | ) | | — | | $ | 1,270,192 | | Susan H. Alexander | | $ | 826,451 | | | $ | 176,553 | | | $ | 392,167 | | | — | | $ | 7,259,032 | | Paul F. McKenzie | | $ | 860,425 | | | $ | 118,612 | | | $ | 2,629 | | | — | | $ | 1,320,342 | |
| | | | | 57 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
| | | | | | | | | | | | | | | | | | Name | | Executive
Contributions
in Last Fiscal
Year(1) | | Company
Contributions
in Last Fiscal
Year(2) | | Aggregate
Earnings
in Last
Fiscal
Year(3) | | Aggregate
Distributions
in Last
Fiscal Year | | Aggregate
Balance at
Last Fiscal Year-End(4) | Michel Vounatsos(5) | | $3,193,708 | | $433,320 | | $518,977 | | — | | $11,835,075 | Michael R. McDonnell | | — | | — | | — | | — | | — | Alfred W. Sandrock, Jr. | | $ 223,260 | | $120,725 | | $235,734 | | — | | $ 4,279,898 | Susan H. Alexander | | $1,000,194 | | $134,365 | | $548,853 | | — | | $10,406,185 | Chirfi Guindo | | $ 677,671 | | $ 58,453 | | $233,703 | | — | | $ 1,833,959 | Jeffrey D. Capello | | — | | $ 72,806 | | $ 480 | | — | | $ 167,923 |
Notes to the 20182020 Non-Qualified Deferred Compensation Table | (1) | The amounts in this column are also included, in part, in columns (c), (e) and/or (f) of the Summary Compensation Table and represent deferral of salary and deferral of payments under our 20182020 annual bonus plan, and deferral of CSPU payments, respectively. |
| (2) | The amounts in this column are also included in column (h) of the Summary Compensation Table for 20182020 as Company contributions to the SSP. |
| (3) | Earnings in excess of 120% of the applicable federal long-term rate are reported in column (g) of the Summary Compensation Table for 20182020 for Mr. Vounatsos ($80,663)264,358), Dr. EhlersSandrock ($5,258),138,444) and Ms. Alexander ($188,056) and Dr. McKenzie ($12,367)311,416). |
| | | | | | | | | 63 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| (4) | The following table lists the compensation deferrals during 20182019 and 20172018 by the NEOs, as reported, where applicable, in the proxy statement for our 20182020 and 2017 Annual Meetings2019 annual meetings of Stockholders:stockholders: |
| | | | | Amounts Previously
Reported as Deferred | | | Amounts Previously
Reported as Deferred | | | | Name | | 2017 | | | 2016 | | | 2019 | | | 2018 | | Mr. Vounatsos | | $ | 1,025,867 | | | $ | 300,000 | | | $ | 1,765,018 | | | $ | 3,071,852 | | Dr. Sandrock | | | $ | 245,492 | | | | — | | Ms. Alexander | | | $ | 838,564 | | | $ | 826,451 | | Mr. Guindo | | | $ | 618,828 | | | | — | | Mr. Capello | | | — | | | | — | | | | — | | | | — | | Dr. Ehlers | | $ | 365,160 | | | $ | 116,250 | | | Ms. Alexander | | $ | 573,897 | | | $ | 259,816 | | | Dr. McKenzie | | $ | 221,128 | | | | — | | |
| | This column also includes Company contributions and compensation earned and deferred in prior years, which was disclosed in our prior proxy statements where applicable, together with earnings on these amounts. |
| (5) | On February 12, 2021, cash-settled PSUs that were elected as deferred by Mr. Vounatsos became vested and credited to the SSP. The earned amount was $891,328. |
Potential Payments Upon Termination or Change in Control Executive Severance Policy Definition of Key Terms Relating to our Executive Severance Policy Our executive severance policy and benefits refer to certain key terms.terms, including cause, change in control, retirement, involuntary employment action and disability. These terms are defined in our 2017 Omnibus Equity Plan. Executive Vice President Arrangements Each of our NEOs, other than Mr. Vounatsos, werewas covered by our executive severance policy in 20182020 under which they werehe or she was eligible to receive the following benefits if certain events occurred during 2018:2020: In the event of a termination of employment other than for cause and other than by reason of the executive’s death or disability, the NEO would be entitled to receive a lump sum severance payment equal to a minimum of 12 months of such NEO’s then base salary and target bonus as then in effect, with an additional 2 months of base salary and target bonus for each full year of service, up to a maximum benefit of 21 months of base salary and target bonus. We refer to the number of months of severance aan NEO is entitled to receive as the “severance period.” If, within two years following a corporate transaction or a corporate change in control, the NEO experiences a termination of employment other than for cause and other than by reason of death or disability or experiences an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s annual base salary plus target annual | | | an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s then-annual base salary plus target annual bonus.bonus as then in effect. These payments are in lieu of any payment in the preceding paragraph.
|
The payment of these severance benefits is conditioned upon execution of an irrevocable release in favor of the Company. The executive severance policy does not pay severance upon a termination for cause, voluntary resignation, retirement or death or disability. In any case where severance is payable under our executive severance policy, our NEOs would also receive continuation of medical, dental and vision insurance benefits until the earlier of the last dateend of the severance period or the date the executive becomes eligible to participate in another employer’s medical, dental and vision insurance plans. NEOs would also be provided up to 12 months of executive-level outplacement services at our cost. Annual Bonus Plan Our annual bonus plan provides for a prorated target bonus payment upon a termination of employment due to the death or disability of the participant. As our annual bonus plan provides for payment of a full bonus to any participant remaining employed on the last day of the plan year, annual bonus amounts are not included in the Potential Post- TerminationPost-Termination Payments Table below. | | | | | 58 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
Mr. Vounatsos’ Arrangements We entered into an employment agreement with Mr. Vounatsos effective January 6, 2017, with2017. The agreement had an initial term endingthat ended on December 31, 2019, at which timeand now the term automatically extends each year for additional12-month periods until the agreement is otherwise terminated in accordance with its terms. | | | | | | | | | 64 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
Under Mr. Vounatsos’ employment agreement, if his employment is terminated by the Company without cause or if he terminates his employment for good reason (referred to in his employment agreement as an involuntary employment action), then he would be entitled to a lump sum payment of cash severance in the amount of one andone-half times his annual base salary and target annual bonus. If, however, such termination of employment occurs within two years following a corporate transaction or a corporate change in control (CIC), then he would be entitled to a lump sum payment of cash severance in the amount of two times his annual base salary and target annual bonus. Mr. Vounatsos would also receive continuation of medical, dental and vision benefits until the earlier of 18 months (24 months if within 2 years of a CIC) following the date his employment terminates or the date upon which he becomes eligible to receive substantially comparable benefits through another employer. In addition, he would be entitled to receive a pro rata portion of his annual cash bonus for the year that such termination occurs based on actual performance or, in the event the termination occurs within two years following a CIC, a pro rata portion of his target annual bonus. Mr. Vounatsos would also be provided executive-level outplacement services for a12-month period following the termination date at our cost. The payment of Mr. Vounatsos’ severance benefits is conditioned upon execution of a general release in favor of the Company. Mr. Capello Arrangements On August 15, 2020, Mr. Capello ceased to be our Executive Vice President and Chief Financial Officer and effective September 15, 2020, Mr. Capello ceased to be employed by us. We paid him the severance benefits payable under our executive severance policy for Executive Vice Presidents and certain additional benefits, as described in the CD&A above under the heading “2020 Hiring- and Transition-Related Compensation Decisions—Arrangements with Mr. Capello.” The amounts received by Mr. Capello in connection with his termination of employment are included in the Summary Compensation Table above in the “All Other Compensation” column. Excise Tax Provisions Before June 2009 we maintained an excise taxgross-up policy for all of our executives, including certain of our NEOs. Under this policy, if payments to these executive officers in the event of a corporate transaction or corporate change in control were subject to the excise tax under Internal Revenue Code Section 4999, we would pay the executive officer an additional amount that equals the amount of the excise tax, plus the income and other payroll taxes arising from our payment of the excise tax amount (280G taxgross-up), so that the executive officer realized the full intended benefit. In June 2009 we changed our excise taxgross-up policy so that newly-hired executives are not eligible for any 280G taxgross-up but may elect to have severance payments reduced to an amount that will not be subject to excise tax. Consistent with this policy, as Ms. Alexander wasand Dr. Sandrock were already eligible for this benefit prior to June 2009, she remainsthey each remained eligible for a 280G tax gross up. No other NEOIn March 2020 Ms. Alexander and Dr. Sandrock each voluntarily waived their rights to this benefit. As a result, no executive officer is currently eligible for this benefit.any such excise tax gross-up. Awards Under Equity Plans Under the provisions of our 2008 Omnibus Equity Plan and our 2017 Omnibus Equity Plan, unless otherwise determined by our C&MD Committee at the time of grant, awards will vest or become exercisable in full immediately prior to an involuntary employment action that occurs within two years following a corporate change in control (i.e., upon a “double trigger”). In the event of a corporate transaction, we can either cause the surviving corporation to assume all equity awards or accelerate their vesting and exercisability immediately before the corporate transaction. If the equity awards are assumed and an executive officer’sa NEO’s employment is terminated in an involuntary employment action within two years following the corporate transaction, the equity awards that are assumed will become fully vested and, if applicable, exercisable. Under our equity plans, any assumed awards that become vested will remain exercisable through the earlier of 12 months from the termination date or the original option expiration date. If the holder of an equity award retires, which is defined under our equity plans as leaving the employment of Biogen after reaching age 55 with 10 consecutive years of service, each then outstanding time-based equity award or earned performance-based equity award not yet vested or exercisable will become immediately vested or exercisable upon such termination at a rate of 50% of the shares unvested at the time of retirement plus an additional 10% of the shares for each full year of service beyond 10 years of service (and performance-based awards would remain eligible to vest based on actual performance). Options vested under these provisions remain exercisable for 36 months from retirement or until the original option expiration date, if sooner. Upon a termination of employment due to death or disability, all unvested time-based equity awards and earned performance-based equity awards vest in full and all unearned performance-based equity awards remain eligible to vest based on actual performance. As of December 31, 2020, Dr. Sandrock and Ms. Alexander were eligible for retirement under our equity plans. | | | | | | | 59 | | 65 | | 
| |  |
| | | 56 | | Executive Compensation Matters (continued) |
Potential Post-Termination Payments Table The following table summarizes the potential payments to each NEO under various termination events. The table assumes that the event occurred on December 31, 2018,2020, for all NEOs.NEOs except for Mr. Capello who separated from the Company during 2020. The calculations use the closing price of our common stock as reported by Nasdaq on December 31, 2018,2020, which was $300.92$244.86 per share. | | Name and Payment Elements(1) (a) | | Retirement(2) (b) | | Qualifying Termination of Employment Not Following a Corporate Transaction or Change in Control (c)(3) | | Qualifying Termination of Employment Following a Corporate Transaction or Change in Control (d)(3) | | Retirement(2) (b) | | Qualifying Termination of Employment Not Following a Corporate Transaction or Change in Control (c)(3) | | Qualifying Termination of Employment Following a Corporate Transaction or Change in Control (d)(3) | Michel Vounatsos(4) | | | | | | | | | | | | | | | | | | | Severance | | | | — | | | | $ | 4,680,000 | | | | $ | 6,240,000 | | | | | — | | | | $ | 5,625,000 | | | | $ | 7,500,000 | | Performance-based RSUs | | | | — | | | | | — | | | | $ | 20,523,378 | | | | | — | | | | | — | | | | $ | 23,217,461 | | Medical, Dental and Vision | | | | — | | | | $ | 29,709 | | | | $ | 39,613 | | | | | — | | | | $ | 29,532 | | | | $ | 39,376 | | Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | — | | | | $ | 4,741,709 | | | | $ | 26,834,991 | | | | | — | | | | $ | 5,686,532 | | | | $ | 30,788,837 | | Jeffrey D. Capello | | | | | | | | | | | Severance | | | | — | | | | $ | 1,487,500 | | | | $ | 2,550,000 | | | Performance-based RSUs | | | | | | | — | | | | $ | 2,736,395 | | | Medical, Dental and Vision | | | | — | | | | $ | 22,611 | | | | $ | 38,761 | | | Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | | Total | | | | — | | | | $ | 1,542,111 | | | | $ | 5,357,156 | | | Michael D. Ehlers | | | | | | | | | | | Michael R. McDonnell | | | | | | | | | | | Severance | | | | — | | | | $ | 1,890,613 | | | | $ | 2,835,920 | | | | | — | | | | $ | 1,487,500 | | | | $ | 2,975,000 | | Performance-based RSUs | | | | — | | | | | — | | | | $ | 7,240,328 | | | | | — | | | | | — | | | | $ | 1,761,269 | | Time-based RSUs | | | | — | | | | | — | | | | $ | 1,736,609 | | | | | — | | | | | — | | | | $ | 1,969,899 | | Medical, Dental and Vision | | | | — | | | | $ | 25,574 | | | | $ | 38,361 | | | | | — | | | | $ | 19,656 | | | | $ | 39,312 | | Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | — | | | | $ | 1,948,187 | | | | $ | 11,883,218 | | | | | — | | | | $ | 1,539,156 | | | | $ | 6,777,480 | | Alfred W. Sandrock, Jr. | | | | | | | | | | | Severance | | | | | — | | | | $ | 2,761,396 | | | | $ | 3,155,881 | | Performance-based RSUs | | | | $ | 6,380,053 | | | | $ | 6,380,053 | | | | $ | 6,380,053 | | Medical, Dental and Vision | | | | | — | | | | $ | 24,943 | | | | $ | 28,506 | | Outplacement(5) | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | $ | 6,380,053 | | | | $ | 9,198,392 | | | | $ | 9,596,440 | | Susan H. Alexander | | | | | | | | | | | | | | | | | | | Severance | | | | — | | | | $ | 2,228,802 | | | | $ | 2,547,202 | | | | | — | | | | $ | 2,422,150 | | | | $ | 2,768,172 | | Performance-based RSUs | | | $ | 4,671,689 | | | | $ | 4,671,689 | | | | $ | 6,673,841 | | | | $ | 5,576,815 | | | | $ | 5,576,815 | | | | $ | 6,196,461 | | Time-based RSUs | | | $ | 852,055 | | | | $ | 852,055 | | | | $ | 1,217,221 | | | Medical, Dental and Vision | | | | — | | | | $ | 23,544 | | | | $ | 26,908 | | | Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | | 280G TaxGross-Up(6) | | | | — | | | | | — | | | | | — | | | Total | | | $ | 5,523,744 | | | | $ | 7,808,090 | | | | $ | 10,497,172 | | | Paul F. McKenzie | | | | | | | | | | | Severance | | | | — | | | | $ | 1,436,926 | | | | $ | 2,155,389 | | | Performance-based RSUs | | | | — | | | | | — | | | | $ | 5,463,914 | | | Time-based RSUs | | | | — | | | | | — | | | | $ | 1,688,161 | | | Medical, Dental and Vision | | | | — | | | | $ | 26,183 | | | | $ | 39,274 | | | | | — | | | | $ | 24,921 | | | | $ | 28,481 | | Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | — | | | | $ | 1,495,109 | | | | $ | 9,378,738 | | | | $ | 5,576,815 | | | | $ | 8,055,886 | | | | $ | 9,025,114 | | Chirfi Guindo | | | | | | | | | | | Severance | | | | | — | | | | $ | 1,446,105 | | | | $ | 1,928,140 | | Performance-based RSUs | | | | | — | | | | | — | | | | $ | 4,575,719 | | Medical, Dental and Vision | | | | | — | | | | $ | 30,269 | | | | $ | 40,359 | | Outplacement(5) | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | | — | | | | $ | 1,508,374 | | | | $ | 6,576,218 | |
Notes to the Potential Post-Termination Payments Table | (1) | In the event of an executive’s death or disability, all outstanding time-based equity awards and earned performance-based equity awards under the Company’sour LTI program will vest in full and all unearned performance-based equity awards will remain outstanding and eligible to vest based on actual performance. The value of such accelerated awards for all NEOs would be the same amount as shown in column (d) for such NEO (based on actual performance estimated as of December 31, 2018)2020). |
| (2) | Dr. Sandrock and Ms. Alexander waswere eligible for potential payments upon retirement at December 31, 2018. Upon retirement, 70%2020. Based on years of any vested CSPUservice, Dr. Sandrock and Ms. Alexander were eligible for accelerated vesting on 100% and 90%, respectively, of their outstanding equity awards would be paid following, if applicable, thesix-month delay required by Section 409Aas of the Internal Revenue Code, 70% of any unvested CSPU awards would vest immediately upon certification of the achievement of the applicable performance goals and would be paid following, if applicable, thesix-month delay required by Section 409A of the Internal Revenue Code.December 31, 2020. Any unvested PSU and MSU awards would, subject to the achievement of any applicable performance goals, remain outstanding and eligible to be earned and vest in accordance with the terms of such awards based on actual performance as to 70%100% for Dr. Sandrock and 90% for Ms. Alexander of the earned shares.PSUs or MSUs, as applicable. The amount listed in column (b) is the estimated value of 70%100% of all unvested awards held by Dr. Sandrock and 90% of all unvested awards held by Ms. Alexander, based on actual performance estimated as of December 31, 2018,2020, for unearned performance-based awards. Based on years of service, Ms. Alexander was eligible for accelerated vesting on 70% of her outstanding awards as of December 31, 2018. |
| (3) | The amounts listed in column (c) and column (d) for Performance-based RSUs for the applicable named executive officers includes the value of applicable unvested awards based, where applicable, on actual performance estimated as of December 31, 2018.2020. |
| | | | | | | | | 66 | | 
| |  |
| | | | 6 | | Executive Compensation Matters (continued) |
| (4) | Pursuant to his employment agreement, upon an involuntary termination by the Company without cause or involuntary employment action not following a corporate transaction or CIC, Mr. Vounatsos is eligible to receive a lump sum payment within 60 days |
| | | | | 60 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued)
|
| of such termination consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary rate and target bonus in effect at the time of termination multiplied by a factor of 1.5, continuation of medical, dental and vision insurance for up to 18 months and up to 12 months of executive outplacement services. Upon an involuntary termination by the Company without cause or an involuntary employment action following a corporate transaction or CIC, Mr. Vounatsos is eligible to receive a lump sum payment within 60 days consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary rate and target bonus in effect at the time of termination multiplied by a factor of 2.0, continuation of medical, dental and vision insurance for up to 24 months and up to 12 months of outplacement services. |
| (5) | The named executive officers are provided outplacement services at a cost of up to $32,000 for the Executive Vice President level. |
(6) | The payments for Ms. Alexander upon a corporate transaction or a corporate change in control on December 31, 2018, would not have been subject to a Section 280G excise tax.
|
CEO Pay Ratio We believe executive pay must be internally consistent and equitable to motivate our employees to create stockholder value, and we are committed to internal pay equity. As discussed earlier in this Proxy Statement, our compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation. We invest in our employees at all levels in the Company by rewarding performance that balances risk and reward, empowering professional growth and development and by offering affordable benefits and programs that meet the diverse needs of our employees. We believe strongly inpay-for-performance, and all of our employees are eligible to participate in our annual bonus plan, our LTI programs and our benefit plans. Our annual bonus plan is consistently maintained for all participants globally, with the same Company performance goals, payout levels (as a percentage of target) and administrative provisions regardless of the participant’s job level, location or function in the Company. We also have a long-term incentive program that provides different forms of awards depending upon an employee’s level but is otherwise consistent throughout the Company. The following is a reasonable estimate, prepared under applicable SEC rules, of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees. Under SEC rules, we used the sameWe determined our median employee as we usedof December 31, 2020, based on a consistently applied compensation measure defined as the sum of base salary, target bonus and LTI target value. We annualized pay for our 2017 pay ratio because we reasonably believe there have been no changes to our employee population or compensation programs that would result in a significant change to our pay ratio disclosure. The methodology used to identify our median employee for our 2017 pay ratio is described in our 2018 proxy statement.employees who commenced employment during 2020. Our median employee is a full-time employee based in the U.S. In October 2017,December 2020, when we determined the median employee, approximately 59%61% of our workforce was based in the U.S. with the remaining approximately 41%39% of our workforce based in the rest of the world. In addition, approximately 98% of our workforce was full-time. For our median employee, annual total compensation was calculated in accordance with the SEC’s rules for the Summary Compensation Table, including salary, bonus, LTI grant date fair value and value of certain benefits provided, including relocation benefits.provided. For our CEO, annual total compensation is equal to the amount included in the “Total” column of the Summary Compensation Table, and our CEO’s annual total compensation for 20182020 was $16,168,646.$18,659,829. The annual total compensation of the median employee, as determined in accordance with the SEC’s rules, for 20182020 was $170,521, including base salary, annual bonus, LTI grant value, and certain benefits includingone-time relocation benefit.$155,378. Based on the foregoing, our estimate of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees was 95120 to 1. This pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules based on our payroll and employment records and the methodology described above. Given the different methodologies, estimates, assumptions and exclusions that other public companies use to determine an estimate of their pay ratio, the estimated ratio reported above should not be used as a basis for comparison between companies. | | | | | | | 61 | | 67 | | 
| |  |
| | | 67 | | Additional InformationOther Management Proposal
|
STOCK OWNERSHIP
The following table and accompanying notes provide information about the beneficial ownership of our common stock by:
each stockholder known by us to be the beneficial owner of more than 5% of our common stock;
each of our named executive officers;
each of our directors and nominees for director; and
all of our directors and executive officers as a group.
Except as otherwise noted, the persons identified have sole voting and investment power with respect to the shares of our common stock beneficially owned. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to the shares. Except as otherwise noted, the information below is as of April 19, 2019 (Ownership Date).
Unless otherwise indicated in the footnotes, the address of each of the individuals named below is: c/o Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142.
| | | | | | | | | | | | | | | | | | | | | | | Name | | Shares
Owned(1) | | | Shares Subject to Options and Stock Units(2) | | | Total Number of Shares Beneficially Owned(1) | | | Percentage of Outstanding Shares(3) | | 5% Stockholders | | | | | | | | | | | | | | | | | BlackRock Inc.(4) 55 East 52nd Street New York, NY 10055 | | | 16,568,963 | | | | — | | | | 16,568,963 | | | | 8.2 | % | The Vanguard Group(5) 100 Vanguard Boulevard Malvern, PA 19355 | | | 15,170,607 | | | | — | | | | 15,170,607 | | | | 7.52 | % | PRIMECAP Management Company(6) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | 14,727,267 | | | | — | | | | 14,727,267 | | | | 7.31 | % | Named Executive Officers | | | | | | | | | | | | | | | | | Michel Vounatsos(7) | | | 16,657 | | | | 3,252 | | | | 19,909 | | | | * | | Jeffrey D. Capello | | | 917 | | | | — | | | | 917 | | | | * | | Michael Ehlers(7) | | | 6,824 | | | | 3,538 | | | | 10,362 | | | | * | | Susan H. Alexander | | | 31,976 | | | | — | | | | 31,976 | | | | * | | Paul F. McKenzie | | | 6,407 | | | | — | | | | 6,407 | | | | * | | Directors | | | | | | | | | | | | | | | | | John R Chiminski | | | — | | | | — | | | | — | | | | — | | Alexander J. Denner(8) | | | 534,687 | | | | 880 | | | | 535,567 | | | | * | | Caroline D. Dorsa | | | 18,172 | | | | 880 | | | | 19,052 | | | | * | | William A. Hawkins | | | — | | | | — | | | | — | | | | — | | Nancy L. Leaming | | | 10,063 | | | | 880 | | | | 10,943 | | | | * | | Jesus B. Mantas | | | — | | | | — | | | | — | | | | — | | Richard C. Mulligan | | | 10,029 | | | | 880 | | | | 10,909 | | | | * | | Robert W. Pangia | | | 17,707 | | | | 880 | | | | 18,587 | | | | * | | Stelios Papadopoulos(9) | | | 29,946 | | | | 1,450 | | | | 31,396 | | | | * | | Brian S. Posner | | | 6,015 | | | | 880 | | | | 6,895 | | | | * | | Eric K. Rowinsky | | | 14,144 | | | | 880 | | | | 15,024 | | | | * | | Lynn Schenk(10) | | | 10,097 | | | | 880 | | | | 10,977 | | | | * | | Stephen A. Sherwin | | | 4,284 | | | | 13,158 | | | | 17,442 | | | | * | | Executive officers and directors as a group (23 persons)(7)(11) | | | 731,239 | | | | 28,438 | | | | 759,677 | | | | * | |
* | Represents beneficial ownership of less than 1% of our outstanding shares of common stock.
|
| | | | | 62 | | | | | | | Proposal 4 – Approve an Amendment to Biogen’s Amended and Restated Certificate of Incorporation, as Amended, to Add a Federal Forum Selection Provision | | | | | | | |
Currently, our Amended and Restated Certificate of Incorporation, as amended (the Certificate of Incorporation), does not include a federal forum selection provision. In response to a decision in the Delaware Supreme Court validating federal forum selection provisions, our Board of Directors reviewed the provision from a legal and policy perspective. In light of this Delaware Supreme Court decision, our Board of Directors has determined that it is in the best interests of our company and our stockholders to seek to include a federal forum selection provision in our Certificate of Incorporation. We are seeking stockholder approval to amend Article XII of our Certificate of Incorporation to provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. In other words, we are seeking to include a federal forum selection provision. Effect of the Amendment Having the federal forum selection provision allows for (a) the consolidation of multi-jurisdiction litigation, (b) avoidance of state court forum shopping and (c) efficiencies in managing the procedural aspects of securities litigation. Given these considerations, our Board of Directors has determined that it is in the best interests of our company and our stockholders that our Certificate of Incorporation be amended to include this federal forum selection provision. There is, however, uncertainty as to whether a court would enforce this provision. Although we are seeking approval of this provision for the reasons cited above, if this provision is approved and implemented, the effects of this amendment may include, but are not limited to, that this provision could discourage claims or limit investors’ ability to bring a claim in a judicial forum that they find favorable. This federal forum selection clause, if it is approved by our stockholders and becomes effective, would be in addition to a provision in our Certificate of Incorporation which provides that the Court of Chancery of the State of Delaware is the exclusive forum for: (1) any derivative action brought on our behalf and (2) any direct action brought by a stockholder against us or any of our directors or officers alleging a violation of the Delaware General Corporation Law, our Certificate of Incorporation or our Bylaws or breach of fiduciary duties or other violation of Delaware decisional law relating to the internal affairs of the corporation. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Accordingly, the exclusive forum provision in our Certificate of Incorporation relating to the Court of Chancery of the State of Delaware does not apply to claims arising under the Exchange Act. Language of Proposed Amendment If approved, the amendment would enable us to amend our Certificate of Incorporation by amending Article XII to read as follows: “ARTICLE XII (a) Exclusive Forum. Unless the Board of Directors or one of its committees otherwise consents to the selection of an alternate forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action brought on behalf of the Corporation and (ii) any direct action brought by a stockholder against the Corporation or any of its directors or officers alleging a violation of the Delaware General Corporation Law, the Corporation’s certificate of incorporation or bylaws or breach of fiduciary duties or other violation of Delaware decisional law relating to the internal affairs of the Corporation; in each case excluding actions in which the Court of Chancery of the State of Delaware concludes that an indispensable party is not subject to the jurisdiction of the Delaware courts and can be subject to the jurisdiction of another court within the United States. (b) Federal Forum. Unless the Corporation consents in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. Any person or entity purchasing or otherwise acquiring any interest in any security of the Corporation shall be deemed to have notice of and consented to this provision.” A copy of the proposed amendment to our Certificate of Incorporation is provided as Appendix B to this proxy statement. | | | | | | | | | 68 | | 
| |  |
| | | 67 | | Additional InformationOther Management Proposal (continued)
|
(1) | The shares described as “owned” are shares of our common stock directly or indirectly owned by each listed person, rounded up to the nearest whole share.
|
(2) | Includes options that are or will become exercisable and RSUs and MSUs that will vest within 60 days of the Ownership Date.
|
(3) | The calculation of percentages is based upon 193,893,397 shares outstanding on the Ownership Date, plus for each of the individuals listed above the shares subject to options and RSUs and MSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and Stock Units.”
|
(4) | Based solely on information as of December 31, 2018, contained in a Schedule 13G/A filed with the SEC by BlackRock Inc. on February 4, 2019, which also indicates that it has sole voting power with respect to 14,695,569 shares and sole dispositive power with respect to 16,568,963 shares.
|
(5) | Based solely on information as of December 31, 2018, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 11, 2019, which also indicates that it has sole voting power with respect to 246,897 shares, sole dispositive power with respect to 14,880,929 shares, shared voting power with respect to 47,063 shares and shared dispositive power with respect to 289,678 shares.
|
(6) | Based solely on information as of December 31, 2018, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 8, 2019, which also indicates that it has sole voting power over 1,696,975 shares and sole dispositive power over 14,727,267 shares.
|
(7) | Includes shares underlying MSUs that will vest within 60 days of the Ownership Date, assuming the maximum possible number of shares that are eligible for vesting on the vesting date. The actual number of shares that will vest on each vesting date will be determined by comparing the price of Biogen common stock on such vesting date to the price on the grant date (i.e., number of vested shares = number of shares at target payout times the[30-day average closing stock price ending on the vesting date divided by the30-day average closing stock price on the grant date]).
|
(8) | Includes 383,858 shares beneficially owned by Sarissa Capital Offshore Master Fund LP, a Cayman Islands exempted limited partnership (Sarissa Offshore), 79,800 shares beneficially owned by Sarissa Capital Catapult Fund LLC, a Delaware limited liability company (Sarissa Catapult) and 61,000 shares beneficially owned by Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital). Sarissa Capital is the investment advisor to certain investment funds, including Sarissa Offshore and Sarissa Catapult. Dr. Denner is the Chief Investment Officer of Sarissa Capital and controls the ultimate general partner of each of Sarissa Capital and Sarissa Offshore and the managing member of Sarissa Catapult. By virtue of the foregoing, Dr. Denner may be deemed to indirectly beneficially own (as that term is defined in Rule13d-3 of the Exchange Act) the shares that those entities beneficially own. Dr. Denner disclaims beneficial ownership of these shares except to the extent of any pecuniary interest therein.
|
(9) | Includes 28,206 shares held in limited liability companies of which Dr. Papadopoulos is the sole manager.
|
(10) | Includes 738 shares held in a trust of which Ms. Schenk is a trustee and 2,362 shares held in a defined benefit plan.
|
(11) | Includes 555,964 shares held indirectly through trusts, funds, defined benefit plans or limited liability companies.
|
Section 16(a) Beneficial Ownership Reporting ComplianceVote Required
Section 16(a)Approval of the Exchange Actamendment to our Certificate of Incorporation requires the affirmative vote of a majority of shares issued and outstanding and entitled to vote on the proposal. Abstentions and broker non-votes will have the effect of votes against this proposal. If a proxy card is signed and returned but no direction is made, the persons named in your proxy will vote your shares “FOR” this proposal.
If our executive officers, directors and greater than 10% stockholders approve the proposed amendment to file initial reportsour Certificate of ownership and changesIncorporation, it will become effective upon filing with the Secretary of ownershipState of our common stock. Asthe State of Delaware of a practical matter,certificate setting forth the amendment, which we assist our directors and executive officers by monitoring transactions and completing and filing Section 16 forms on their behalf. Based solely on information provided to us by our directors and executive officers, we believe that during 2018 all such parties complied with all applicable filing requirements.anticipate doing as soon as practicable following stockholder approval. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE APPROVAL OF AN AMENDMENT TO BIOGEN’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION, AS AMENDED, TO ADD A FEDERAL FORUM SELECTION PROVISION | | | | | | | 63 | | 69 | | 
| |  |
| | | 68 | | Additional InformationStockholder Proposals
|
| | | | | | | | | | | | Proposal 5 – Stockholder Proposal Requesting a Report on Biogen’s Lobbying Activities | | | | | | | |
Mr. James McRitchie and Ms. Myra K. Young (whom we sometimes refer to as the Proposal 5 Proponents) have notified us that they intend to submit the following proposal for consideration at the Annual Meeting. The Proposal 5 Proponents have indicated that they beneficially own 25 shares of our common stock. We will provide their address promptly upon a stockholder’s oral or written request. The Proposal 5 Proponents are responsible for the content of the proposal, for which we and our Board of Directors accept no responsibility. The proposal will be voted on at the Annual Meeting if the Proposal 5 Proponents, or a qualified representative, is present at the Annual Meeting and submits the proposal for a vote. Our statement in opposition follows the proposal. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE AGAINST THE APPROVAL OF THIS STOCKHOLDER PROPOSAL. Proposal 5 – Disclose Lobbying Expenditures Full disclosure of Biogen’s direct and indirect lobbying activities and expenditures is needed to assess whether Biogen’s lobbying is consistent with its expressed goals and stockholder interests. Resolved: Stockholders of Biogen request preparation of an annual report disclosing: | 1. | Company policy and procedures governing lobbying, both direct and indirect, and grassroots lobbying communications. |
| 2. | Payments by Biogen used for (a) direct or indirect lobbying or (b) grassroots lobbying communications, in each case including the amount of the payment and the recipient. |
| 3. | Biogen’s membership in and payments to any tax-exempt organization that writes and endorses model legislation. |
| 4. | Description of management’s decision-making process and the Board’s oversight for making payments described in section 2 above. |
For purposes of this proposal, “grassroots lobbying communication” is communication directed to the general public that (a) refers to specific legislation or regulation, (b) reflects a view on legislation or regulation and (c) encourages the communication recipient to take action with respect to legislation or regulation. “Indirect lobbying” is lobbying engaged in by a trade association or other organization of which Biogen is a member. Both “direct and indirect lobbying” and “grassroots lobbying communications” include efforts at local, state and federal levels. The report shall be presented to the Corporate Governance Committee and posted on Biogen’s website. Supporting Statement Biogen spent $20,761,680 from 2010 – 2019 on federal lobbying. This does not include state lobbying, where Biogen also lobbies but disclosure is uneven or absent. For example, Biogen lobbied in at least 18 state in 2019 (followthemoney.org). Biogen’s trade association and social welfare group disclosure has gaps for memberships and spending. Biogen discloses trade associations receiving more than $25,000 and amounts used to lobby, including the Pharmaceutical Research and Manufacturers of America (PhRMA). Biogen’s disclosure fails to capture memberships in and support for the Healthcare Leadership Council, which spent $660,000 lobbying in 2019, National Pharmaceutical Council, and Alliance for Patient Access (AfPA), a social welfare group. And Biogen reported $8,916,842 in PhRMA lobbying payments, while reporting $5,136,680 in federal lobbying for 2018 and 2019. Therefore, at least $3,780,162 of Biogen’s payments were used for non-federal lobbying and grassroots. Grassroots lobbying is not reported at the federal level under the Lobbying Disclosure Act. State-level disclosure is uneven or absent. We are concerned Biogen’s payments to third party groups are potentially used for undisclosed grassroots lobbying. For example, PhRMA, with 2018 revenues of $459 million, gave millions to “dark money” social welfare groups, which “advocated policies favored by drugmakers”.1 PhRMA has also been linked to “pharma-backed astroturf group” Coalition Against Socialized Medicine.2 AfPA has been described as “a front group established solely to do the bidding of industry.”3 We urge Biogen to expand its lobbying disclosure. | | | | | | | | | 70 | | 
| |  |
| | | | 8 | | Stockholder Proposals (continued) |
| 1 | https://www.opensecrets.org/news/2019/11/big-pharma-bankrolled-conservative-groups-tax-returns-show/ |
| 2 | https://prospect.org/health/pharma-backed-astroturf-group-drug-prices/ |
| 3 | https://www.healthnewsreview.org/2017/10/non-profit-alliance-patient-access-uses-journalists-politicians-push-big-pharmas-agenda/ |
Company Statement in Opposition Our Board of Directors recommends that stockholders vote AGAINST this stockholder proposal for the following reasons: Lobbying priorities and disclosure. Our lobbying priorities consider the interests of patients, our company, stockholders, employees and other stakeholders. In 2020 our federal lobbying priorities focused on healthcare, access to prescription drugs and patent protection, all of which directly impact our business and stockholder value. Our Board of Directors and its committees play an important role in our public policy engagement and have oversight responsibilities for these activities. The Corporate Governance Committee is responsible for reviewing annually the Company’s actions related to our lobbying priorities and activities, including associations with certain trade and/or legislative organizations. We value transparency in this process and appreciate the need for disclosure of our political activity to promote ethical corporate governance and confidence in the democratic process. Our corporate political contributions are disclosed in accordance with applicable federal and state campaign finance laws and in our semi-annual Political Contributions Disclosures. All Political Contributions Disclosures as well as our United States Political Contributions Policy are available on our website at https://investors.biogen.com/governance/political-contributions-disclosures. In the U.S., we comply with important federal and state lobbying registration and disclosure laws. Our current disclosures, detailing our federal lobbying priorities, are fully compliant with the Federal Lobbying Disclosure Act and the Honest Leadership and Open Government Act and are filed quarterly with the U.S. House of Representatives and the U.S. Senate. Included in the report is the total amount spent on federal lobbying activity for the quarter, which includes the percentage of our dues to trade associations spent on federal lobbying activity, payments to outside consultants and the time spent by Biogen colleagues on federal lobbying activity. Our disclosures on federal lobbying activities may be viewed at: https://soprweb.senate.gov/index.cfm?event=selectfields. We are also fully compliant with state registration and reporting requirements in all of the states where we operate. These state reports are publicly available at the appropriate state agency or on the state’s public web site. Moreover, our lobbying activities are subject to robust internal procedures designed to align these efforts with our public policy priorities and applicable law. We have a robust training and reporting program in place to ensure colleague compliance. Our engagement with lawmakers and trade and industry organizations. We operate in a highly regulated and competitive industry and continue to face significant legislative and regulatory challenges. It is essential that we actively engage with lawmakers and trade and industry organizations to help build constructive discourse in the political and regulatory environment in support of our business priorities. To enhance these efforts, we are members of several industry and trade groups. We believe value exists in making sure our positions on issues important to us and our industry are communicated and understood within these organizations. A listing of our current memberships with several industry and trade groups is available on our website at: https://investors.biogen.com/governance/political-contributions-disclosures. Annual evaluation and oversight of our support for trade and industry groups. We annually evaluate our support of industry and trade groups based on these organizations’ expertise in healthcare policy and advocacy and support of key issues of importance to us. In addition to their positions on healthcare policy issues, we realize these organizations may engage in a broad range of other issues that extend beyond the scope of issues of primary importance to us. If concerns arise about an issue, we are able to convey our concerns, as appropriate, through our colleagues who serve on the boards and committees of these groups. Decisions to provide funding are based on an organization’s support of issues that impact our industry, including advancing biomedical research, healthcare innovation, advocating for protecting intellectual property rights and access to medicines. Summary. We believe our enhanced disclosures provide transparency into and accountability regarding lobbying activities that is responsive to the proposal and that the proposal’s additional reporting obligation would be burdensome and unnecessary. | | | | | | | | | 71 | | 
| |  |
| | | | 8 | | Stockholder Proposals (continued) |
| | | | | | | | | | | | Proposal 6 – Stockholder Proposal Requesting a Report on Biogen’s Gender Pay Gap | | | | | | | |
Proxy Impact (whom we sometimes refer to as the Proposal 6 Proponent) has notified us that it intends to submit the following proposal for consideration at the Annual Meeting. Proxy Impact has represented that it is authorized by owners of 50 shares of our common stock to submit this proposal. We will provide the Proposal 6 Proponent’s address promptly upon a stockholder’s oral or written request. The Proposal 6 Proponent is responsible for the content of the proposal, for which we and our Board accept no responsibility. The proposal will be voted on at the Annual Meeting if the Proposal 6 Proponent, or a qualified representative, is present at the Annual Meeting and submits the proposal for a vote. Our statement in opposition follows the proposal. OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE AGAINST THE APPROVAL OF THIS STOCKHOLDER PROPOSAL. Whereas: The 2017 U.S. Census data on median earnings for full-time, year-round workers found that women made 80 percent of that of their male counterparts. The gap for African America and Latina women is 60 percent and 55 percent. At the current rate, women will not reach pay parity until 2059. Mercer finds actively managing pay equity “is associated with higher current female representation at the professional through executive levels and a faster trajectory to improved representation.” Research from Morgan Stanley, McKinsey, and Robeco Sam suggests more gender diverse leadership leads to superior stock price performance and return on equity. McKinsey states, “the business case for the advancement and promotion of women is compelling.” Best practices include “tracking and eliminating gender pay gaps.” Assessing if a company has a gender pay gap requires analyzing both equal pay and equal opportunity. This is most commonly done using adjusted and unadjusted (median) pay data. Median pay data is the key metric used by the Organization for Economic Cooperation and Development, the World Economic Forum, and the U.S. Department of Labor, among others. Since 2018, the UK has mandated disclosures of both adjusted and unadjusted (median) gender pay data, demonstrating that the publication of such data is feasible and informative. Biogen UK provides an annual gender pay report that reports mean and median gender pay gap and bonus gap, and pay quartiles. The Biogen UK 2019-2020 gender pay gap report states that it has a sixteen percent mean and eight percent median hourly wage gap, and a twenty-eight percent mean and twenty-five percent median bonus pay gap. Biogen does not report on the gender pay gap for its U.S. employees. Investors seek quantitative, comparable data to understand the effectiveness of Biogen U.S. pay gap policies. Regulatory risks associated with pay equity exist. The Paycheck Fairness Act, pending in Congress, would improve company-level transparency and strengthen penalties for equal pay violations. Massachusetts, California, New York and Maryland have enacted significant changes to their equal pay laws. Companies would be well served by understanding the equity attributes of their pay, at all levels of the corporation, by gender as well as other facets of diversity, such as race and ethnicity. Leading large-cap companies across industry sectors including Apple, Starbucks and Bank of New York Mellon, among others, have publicly committed to pay equity and published the results of gender pay assessments. Resolved: Shareholders request that Biogen publish annually, quantitative data assessing Biogen’s gender pay gap, at reasonable expense and excluding proprietary information. A report adequate for investors to assess company strategy and performance, including relative opportunities for women to attain higher paying positions in the company, would include the percentage mean and median pay gap between all male and female employees, across race and ethnicity where appropriate, and would include base, bonus and equity compensation. | | | | | | | | | 72 | | 
| |  |
| | | | 8 | | Stockholder Proposals (continued) |
Company Statement in Opposition Our Board of Directors recommends that stockholders vote AGAINST this stockholder proposal for the following reasons: We are committed to ensuring that our employees receive equal pay for equal work. Our Board of Directors and its committees oversee elements of our corporate culture, including human capital management, associated with their respective areas of responsibility. Our Board of Directors recognizes the value of Biogen’s colleagues and the need for the Company to build and sustain a culture where colleagues of diverse backgrounds and abilities contribute their unique viewpoints and perspectives to all aspects of the business. Our Board of Directors is also committed to rewarding our employees equitably. Our pay equity study confirms equitable pay practices. We establish the components and ranges of compensation based on market and benchmark data. Within this context, we strive to pay all employees equitably within a reasonable range, taking into consideration factors such as role, function, market data, internal equity, job location, relevant experience and individual, business unit and company performance. In addition, we are committed to providing flexible benefits designed to allow our diverse global workforce to have reward opportunities that meet their varied needs so that they are inspired to perform their best on behalf of patients and stockholders each day. We regularly review our compensation practices and analyze the equity of compensation decisions for individual employees and our workforce as a whole. If we identify employees with pay gaps, we review and take appropriate action to ensure fidelity between our stated philosophy and actions. To better understand our pay equity performance, in 2020 we had an external consultant complete a global pay equity analysis to help us analyze comparable roles and evaluate whether gender impacted compensation at Biogen. In the U.S., where the law permits the collection of race data, we also included race in the analysis, consistent with our commitment to racial equity. Overall, 85% of our global workforce was included in at least one analysis. Individuals not included in the analysis were either single-incumbent roles or in homogenous groups (by gender or by race), and therefore did not need to be analyzed. We analyzed our employees’ pay relative to race, gender, geography, responsibilities, level, performance and a wide range of other criteria. The pay equity analysis showed that the compensation of 99.7% of those analyzed was equitable. For the remaining 0.3% (17 employees) we made appropriate adjustments. The robust analysis confirmed that fairness and equity are embedded in our compensation practices around the globe. We will continue to regularly review our diversity and our compensation philosophy, ensure employees understand our total compensation practices and provide training for managers and leaders to prevent bias during hiring, compensation decisions and performance management. Summary. We are confident that our current compensation philosophy, policies and practices regarding pay equity and opportunity parity as well as our recent commitment and our current disclosure practices make this proposal unnecessary. | | | | | | | | | 73 | | 
| |  |
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS Our Code of Business Conduct (Values in Action), Corporate Governance Principles, Related Person Transaction Policy and Conflict of Interest Policy set forth our policies and procedures for the review and approval of transactions with related persons, including transactions that would be required to be disclosed in this Proxy Statement in accordance with SEC rules. In circumstances where one of our directors or executive officers, or a family member, has a direct or indirect material interest in a transaction involving Biogen, our Corporate Governance Committee must review and approve all such proposed transactions or courses of dealing. In determining whether to approve or ratify a transaction with a related person, among the factors our Corporate Governance Committee may consider (as applicable) are: the business reasons for entering into the transaction; the size of the transaction and the nature of the related person’s interest in the transaction; whether the transaction terms are as favorable to us as they would be to an unaffiliated third party; whether the transaction terms are more favorable to the related person than they would be to an unaffiliated third party; the availability of alternative sources for comparable products, services or other benefits; whether the transaction would impair the independence or judgment of the related person in the performance of his or her duties to us; fornon-employee directors, whether the transaction would be consistent with Nasdaq’s requirements for independent directors; whether the transaction is consistent with our Conflict of Interest Policy, which prohibits related persons and others from having a financial interest in any competitor, customer, vendor or supplier of ours; the related person’s role in arranging the transaction; the potential for the transaction to be viewed as representing or leading to an actual or apparent conflict of interest; and any other factors that our Corporate Governance Committee deems appropriate. Our Code of Business Conduct, which sets forth legal and ethical guidelines for all of our directors and employees, states that directors, executive officers and employees must avoid relationships or activities that might impair their ability to make objective and fair decisions while acting in their Company roles. There Other than as noted below, there are no relationships or transactions with related persons that are required to be disclosed in this Proxy Statement under SEC rules. Dr. Sandrock has a daughter employed by us in a non-executive position outside of the Research & Development organization who received approximately $220,000 in total compensation in 2020. Consistent with our Related Person Transaction Policy as described above, our Corporate Governance Committee reviewed this matter. On January 5, 2021, we entered into an exclusive license agreement (the License Agreement) with Sana. Dr. Mulligan currently serves as the Head of SanaX, the research arm of Sana, and Executive Vice Chairman of Sana. Under the License Agreement, we granted Sana an exclusive sublicense (except as to Biogen) of certain riboswitch-based technology licensed to us by Baylor College of Medicine (the Baylor Technology). We have a non-exclusive license to any improvements made by Sana to the Baylor Technology for use by us in our core and emerging growth areas (the Biogen Field). We have a right of first refusal should Sana decide to seek a collaboration for any program in the Biogen Field. Sana will pay us a royalty in the low single digits on net sales of any products developed under the License Agreement (the Sana Products) and will share half of the patent maintenance costs related to the Baylor Technology. The License Agreement will continue until the later of (a) such time as all of Sana’s payment obligations related to all Sana Products expire and (b) the expiration of the royalty terms, as defined in the License Agreement, for all Sana Products. Consistent with our Related Person Transaction Policy as described above, our Corporate Governance Committee reviewed this matter. | | | | | | | 64 | | 74 | | 
| |  |
| | | 69 | | Additional Information (continued) |
EQUITY COMPENSATION PLAN INFORMATION The following table provides information as of December 31, 2018,2020, about: the number of shares of common stock subject to issuance upon exercise of outstanding options and vesting of RSUs, MSUs and PSUs under plans adopted and assumed by us; the weighted-average exercise price of outstanding options under plans adopted and assumed by us;us (assuming target performance); and
the number of shares of common stock available for future issuance under our active plans: our 2017 Omnibus Equity Plan, ourNon-Employee Directors Equity Plan and our 2015 Employee Stock Purchase Plan. | | | Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights (a) | | Weighted-average Exercise Price of Outstanding Options and Rights(1) (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(2) (c) | | Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights (a) | | Weighted-average Exercise Price of Outstanding Options and Rights (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(1) (c) | Equity compensation plans approved by stockholders | | 1,308,264 | | $ 53.82 | | 20,564,534 | | 1,492,565 | | — | | 18,100,439 | Equity compensation plans not approved by stockholders | | — | | — | | — | | — | | — | | — | Total | | 1,308,264 | | $ 53.82 | | 20,564,534 | | 1,492,565 | | — | | 18,100,439 |
| (1) | The weighted-average exercise price includes all outstanding stock options but does not include RSUs MSUs or PSUs, which do not have an exercise price.
|
(2) | Of these shares, (a) 14,127,58112,116,477 remain available for future issuance under our 2017 Omnibus Equity Plan, (b) 703,425665,955 remain available for future issuance under ourNon-Employee Directors Equity Plan and (c) 5,733,5285,318,007 remain available under our 2015 Employee Stock Purchase Plan. In addition to shares issuable upon the exercise of options or rights, the shares under our 2017 Omnibus Equity Plan and ourNon-Employee Directors Equity Plan may also be issued other than upon such exercise. |
| | | | | | | 65 | | 75 | | 
| |  |
| | | 69 | | Additional Information (continued) |
MISCELLANEOUS Stockholder Proposals Stockholder proposals submitted pursuant to Exchange Act Rule14a-8 and intended to be presented at our 20202022 annual meeting of stockholders must be received by our Secretary no later than December 30, 2019,24, 2021, to be eligible for inclusion in our proxy statement and form of proxy relating to that meeting. A stockholder proposal submitted outside the processes of Rule14a-8 and not for inclusion in our proxy statement for the 20202022 annual meeting of stockholders will be ineligible for presentation at the meeting unless the stockholder gives timely notice of the proposal in writing to our Secretary at our principal executive offices and otherwise complies with the provisions of our Bylaws. To be timely, our Bylaws provide that we must have received the stockholder’s notice no later than March 21, 2020,4, 2022, and no earlier than February 20, 2020.2, 2022. However, if the date of the 20202022 annual meeting of stockholders is more than 30 days before or more than 60 days after the first anniversary of the Annual Meeting, we must receive the stockholder’s notice not earlier than the close of business on the 120th day before the 20202022 annual meeting of stockholders and not later than the close of business on the later of (1) the 90th day before the 20202022 annual meeting of stockholders and (2) the 10th day following the day on which public announcement of the date of the 20202022 annual meeting of stockholders is first made. All stockholder proposals for our 20202022 annual meeting of stockholders should be sent to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. Other Stockholder Communications Generally, stockholders who have questions or concerns should contact our Investor Relations department at (781)464-2442. However, stockholders who wish to communicate directly with our Board of Directors, or any individual director, should direct questions in writing to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. Communications addressed in this manner will be forwarded directly to our Board of Directors or named individual director(s). Incorporation by Reference Notwithstanding anything to the contrary set forth in any of our previous filings under the securities laws that might incorporate future filings, including this Proxy Statement, in whole or in part, the Compensation Committee Report, the Audit Committee Report, the content ofwww.biogen.com, including the charters of the committees of our Board of Directors, Corporate Governance Principles, Related Person Transaction Policy, Conflicts of Interest Policy, Code of Business Conduct, Certificate of Incorporation and Bylaws, included or referenced in this Proxy Statement shall not be incorporated by reference into any such filings. Copies of Annual Meeting Materials Some banks, brokers and other nominee record holders may be participating in the practice of householding proxy statements and annual reports. This means that, unless you have instructed otherwise, only one copy of this Proxy Statement, Annual Report or Notice of Internet Availability of Proxy Materials, as applicable, may have been sent to multiple stockholders in your household.We will promptly deliver a separate copy of any of these documents without charge to you if you write or call Investor Relations, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142,(781) 464-2442464-2442..If you want to receive separate copies of our proxy statement, annual report or Notice of Internet Availability of Proxy Materials, as applicable, in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at the above address or phone number. Manner and Cost of Proxy Solicitation Biogen pays the cost of soliciting proxies. In addition to solicitation by mail, our directors, officers and employees may contact you in person, by telephone or by email or other electronic means. None of our directors, officers or employees will receive additional compensation for soliciting you. We will reimburse brokerage houses, banks, custodians and other nominees and fiduciaries forout-of-pocket expenses incurred in forwarding our proxy solicitation materials to, and obtaining instructions relating to such materials from, beneficial owners of our common stock. Georgeson LLC, New York, New York, has been retained to assist us in the solicitation of proxies at a fee estimated not to exceed $11,000.$12,650. | | | | | | | 66 | | 76 | | 
| |  |
APPENDIX A GAAP toNon-GAAP Reconciliation Diluted Earnings Per Share and Net Income Attributable to Biogen Inc. (unaudited, $ in millions, except per share amounts) | | | | | For the Twelve Months Ended | | For the Twelve Months Ended | | | | | December 31, 2018 | | December 31, 2017 | | December 31, 2020 | | December 31, 2019 | GAAP earnings per share – Diluted | | | $21.58 | | | | $11.92 | | | | $24.80 | | | | $31.42 | | Adjustments to GAAP net income attributable to Biogen Inc. (as detailed below) | | | 4.62 | | | | 9.89 | | | | 8.90 | | | | 2.15 | | Non-GAAP earnings per share – Diluted | | | $26.20 | | | | $21.81 | | | | $33.70 | | | | $33.57 | |
| | | | | | | | | | | | | | | | For the Twelve Months Ended | | | | | | | December 31, 2018 | | December 31, 2017(1) | GAAP net income attributable to Biogen Inc. | | | | $4,430.7 | | | | | $2,539.1 | | Adjustments: | | | | | | | | | | | Amortization of acquired intangible assetsA, B | | | | 747.3 | | | | | 814.7 | | Acquiredin-process research and development | | | | 112.5 | | | | | 120.0 | | Research and developmentC | | | | 10.0 | | | | | — | | (Gain) loss on fair value remeasurement of contingent considerationD | | | | (12.3) | | | | | 62.7 | | Premium paid on purchase of Ionis common stockE | | | | 162.1 | | | | | — | | (Gain) loss on equity security investments | | | | (128.0) | | | | | — | | Net distribution to noncontrolling interestsF | | | | 43.7 | | | | | 132.4 | | Restructuring, business transformation and other cost saving initiatives: | | | | | | | | | | | 2017 corporate strategy implementationG | | | | 10.9 | | | | | 18.5 | | Restructuring chargesG | | | | 12.0 | | | | | 0.9 | | Hemophilia business separation costs | | | | — | | | | | 19.2 | | Income tax effect related to reconciling items | | | | (146.6) | | | | | (235.7) | | Elimination of deferred tax assetH | | | | 10.6 | | | | | — | | Tax reformI | | | | 124.9 | | | | | 1,173.6 | | Non-GAAP net income attributable to Biogen Inc. | | | | $5,377.8 | | | | | $4,645.4 | |
| | | | | | | | | | | | | | | | For the Twelve Months Ended | | | | | | | December 31, 2020* | | December 31, 2019 | GAAP net income attributable to Biogen Inc. | | | | $4,000.6 | | | | | $5,888.5 | | Adjustments: | | | | | | | | | | | Acquisition and divestiture related costs: | | | | | | | | | | | Amortization of acquired intangible assetsA | | | | 464.8 | | | | | 489.9 | | Acquired in-process research and development | | | | 75.0 | | | | | — | | (Gain) loss on fair value remeasurement of contingent considerationA | | | | (86.3 | ) | | | | (63.7 | ) | Loss on divestiture of Hillerød, Denmark manufacturing operationsB | | | | (92.5 | ) | | | | 55.3 | | Net distribution to noncontrolling interests | | | | 0.3 | | | | | — | | Stock option expense | | | | — | | | | | 26.2 | | Acquisition-related transaction and integration costs | | | | 19.5 | | | | | 27.9 | | Accelerated share-based compensation expense | | | | — | | | | | 6.7 | | Subtotal: Acquisition and divestiture related costs | | | | 380.8 | | | | | 542.3 | | Restructuring, business transformation and other cost saving initiatives: | | | | | | | | | | | 2017 corporate strategy implementation | | | | — | | | | | 3.5 | | Restructuring charges | | | | — | | | | | 1.5 | | Other cost saving initiatives | | | | 2.8 | | | | | — | | Subtotal: Restructuring, business transformation and other cost saving initiatives | | | | 2.8 | | | | | 5.0 | | (Gain) loss on equity security investments | | | | (693.9) | | | | | (200.2) | | Sangamo upfront payment and premium paid on the purchase of Sangamo common stockC | | | | 208.2 | | | | | — | | Denali upfront payment and premium paid on the purchase of Denali common stockD | | | | 601.3 | | | | | — | | Sage upfront payment and premium paid on the purchase of Sage common stockE | | | | 1,084.0 | | | | | — | | Premium paid on early debt redemption | | | | 9.4 | | | | | — | | Valuation allowance associated with deferred tax assetsF | | | | 90.3 | | | | | — | | Income tax effect related to reconciling items | | | | (287.9) | | | | | 31.3 | | Swiss tax reformG | | | | — | | | | | (54.3) | | Amortization included in Equity in loss of investee, net of tax | | | | 40.0 | | | | | 78.2 | | Non-GAAP net income attributable to Biogen Inc. | | | | $5,435.6 | | | | | $6,290.8 | |
| * | Beginning in the third quarter of 2020 material upfront payments associated with significant collaboration and licensing arrangements are excluded from Non-GAAP R&D expense in order to better reflect the Company’s core operating performance. Full year Non-GAAP results reflect this change as the $125.0 million upfront payment related to the collaboration with Sangamo Therapeutics, Inc. in the second quarter of 2020 has been excluded from Non-GAAP R&D expense. |
| | | | | | | | | A-1 | | 
| |  |
Appendix A (continued) Free Cash Flow Reconciliation (unaudited, $ in millions) | | | | | | | | | | | For the Twelve
Months Ended | | | | December 31, 20182020
| Net cash flows provided by operating activities | | | | $ 6,187.74,229.8 | | Net cash used in investing activities | | | | (608.6) | | Net cash used in financing activities | | | | (5,272.7) | | Net increase (decrease) in cash and cash equivalents | | | $ | (1,651.5) | | Net cash provided by operating activities | | | | $ 4,229.8 | | Purchases of property, plant and equipment (Capital Expenditures) | | | | (770.6)
| | Contingent consideration related to Fumapharm AG acquisition
| | | | (1,500.0)(424.8)
| | Free Cash FlowFlow^ | | | | $ 3,917.13,805.0 | |
(1)^ | On February 1, 2017, we completed thespin-off of our hemophilia business. Our consolidated results ofFree cash flow is defined as net cash flow from operations reflect the financial results of our hemophilia business through January 31, 2017.less capital expenditures.
|
| A | In January 2017 we entered into a settlementAmortization and license agreement among Biogen Swiss Manufacturing GmbH, Biogen International Holding Ltd., Forward Pharma A/S (Forward Pharma) and certain related parties, which was effective February 1, 2017. Pursuant to this
|
| | | | | A-1 | |  | |  |
Appendix A(continued)
| agreement, we obtained U.S. and rest of world licenses to Forward Pharma’s intellectual property, including Forward Pharma’s intellectual property related to TECFIDERA. In exchange, we paid Forward Pharma $1.25 billion in cash, of which $795.2 million was recognized as an intangible asset in the first quarter of 2017. |
| We have two intellectual property disputes with Forward Pharma, one in the U.S. and one in the European Union, concerning intellectual property related to TECFIDERA.
|
| In March 2017 the U.S. intellectual property dispute was decided in our favor. Forward Pharma appealed to the U.S. Court of Appeals for the Federal Circuit. We evaluated the recoverability of the U.S. asset acquired from Forward Pharma and recorded a $328.2 million impairment charge in the first quarter of 2017 to adjust the carrying value of the acquired U.S. asset to fair value reflecting the impact of the developments in the U.S. legal dispute and continued to amortize the remaining net book value of the U.S. intangible asset in our consolidated statements of income utilizing an economic consumption model. The U.S. Court of Appeals for the Federal Circuit upheld the U.S. Patent and Trademark Office’s March 2017 ruling and in January 2019 denied Forward Pharma’s petition for rehearing. We evaluated the recoverability of the U.S. asset based upon these most recent developments and recorded a $176.8 million impairment charge in the fourth quarter of 2018 to reduce the remaining net book value of the U.S. asset to zero.
|
| In March 2018 the European Patent Office (EPO) revoked Forward Pharma’s European Patent No. 2 801 355. Forward Pharma has filed an appeal to the Technical Boards of Appeal of the EPO and the appeal is pending. Based upon our assessment of this ruling, we continue to amortize the remaining net book value of the rest of world intangible asset in our consolidated statements of income utilizing an economic consumption model.
|
| Amortization of acquired intangible assets for the twelve months ended December 31, 2017, also includes2020, reflects the impact of the impairment charges related to timrepigene emparvovec (BIIB111), which was obtained as part of the Nightstar Therapeutics plc acquisition, and cinpanemab (BIIB054) as well as a $31.2$19.3 millionpre-tax impairment charge related to one of our acquired andin-licensed rights and patents intangible asset associated with ZINBRYTA after the initiation of an European Medicines Agency review (referred to as an Article 20 Procedure) of ZINBRYTA following the report of a case of fatal fulminant liver failure, as well as four cases of serious liver injury.
|
B | Amortization of acquired intangible assets for the twelve months ended December 31, 2018, includes the impact of impairment charges totaling $189.3 million related to certainin-process research and development (IPR&D) assetsintangible assets. During the fourth quarter of 2020 we experienced third-party manufacturing delays for BIIB111 and determined that forecasted costs associated with advancing the program through development and commercialization will exceed our vixotrigine (BIIB074) program.original estimates. We reassessed the fair value of the program based on these changes in assumptions and determined that the program was partially impaired. We recognized an impairment charge of approximately $115.0 million during the fourth quarter of 2020.
|
| | During the third quarterIn February 2021 we announced that we discontinued development of 2018 we completed aBIIB054 in Parkinson’s disease as our Phase 2b study of vixotrigine for the treatment of painful lumbosacral radiculopathy (PLSR). The2 SPARK study did not meet its primary or secondary efficacy endpoints; therefore,endpoints. Although we discontinued developmentmade this determination in February 2021, it was based on conditions that existed as of vixotrigine for the treatment of PLSR andDecember 31, 2020. As a result, we recognized an impairment charge of approximately $60.0$75.4 million during the thirdfourth quarter of 20182020 to reduce the fair value of the related IPR&D intangible asset to zero. In addition, we delayedWe also adjusted the initiation of the Phase 3 studies of vixotrigine for the treatment of trigeminal neuralgia (TGN) as we awaited the outcome of ongoing interactions with the U.S. Food and Drug Administration (FDA) regarding the design of the Phase 3 studies, a more detailed review of the data from the Phase 2b study of vixotrigine for the treatment of PLSR and insights from the Phase 2 study of vixotrigine for the treatment of small fiber neuropathy. We reassessed the fair value of our vixotrigine program forcontingent consideration obligation related to BIIB054 resulting in a gain of $51.0 million in the treatment of TGN using reduced expected lifetime revenues, higher expected clinical development costs and a lower cumulative probability of success and, as a result of that assessment, we recognized an impairment charge of $129.3 million during the thirdfourth quarter of 2018 to reduce the fair value of the IPR&D intangible asset associated with our vixotrigine program for the treatment of TGN to $41.8 million.2020.
|
C | GAAP researchAmortization and development expenseimpairment of acquired intangible assets for the twelve months ended December 31, 2018, include2019, reflects the impact of a $10.0$215.9 million contingent consideration payment accrued in relationimpairment charge related to certain IPR&D assets associated with the acquisitionPhase 2b study of an asset.
|
D | DuringBG00011 (STX-100) for the potential treatment of idiopathic pulmonary fibrosis, which was discontinued during the third quarter of 2018, we2019. We also adjusted the fair value of our contingent consideration obligations related to our vixotrigine program for the treatment of TGN to reflect the lower cumulative probabilities of success, which resultedBG00011 resulting in a gain of $89.6$61.2 million in the third quarter.
|
| B | In August 2019 we completed the sale of all of the outstanding shares of our subsidiary that owned our biologics manufacturing operations in Hillerød, Denmark to FUJIFILM Corporation. Upon the closing of this transaction, we received approximately $881.9 million in cash, which may be adjusted based on other contractual terms, which are discussed below. |
| In connection with this transaction we recognized a total net loss of approximately $164.4 million in our consolidated statements of income. This loss included a pre-tax loss of $95.5 million, which was recorded in loss on divestiture of Hillerød, Denmark manufacturing operations. The loss recognized was based on exchange rates and business conditions on the closing date of this transaction, and included costs to sell our Hillerød, Denmark manufacturing operations of approximately $11.2 million and our estimate of the fair value of an adverse commitment of approximately $114.0 million associated with the guarantee of future minimum batch production at the Hillerød facility. The value of this adverse commitment was determined using a probability-weighted estimate of future manufacturing activity. We also recorded a tax expense of $68.9 million related to this transaction. During the fourth quarter of 2019 we recorded a $40.2 million reduction in our estimate of the future minimum batch commitment utilizing our current manufacturing forecast, which reflects the impact of forecasted batches of aducanumab, an investigational treatment for Alzheimer’s disease, resulting in a reduction in the pre-tax loss on divestiture from $95.5 million to $55.3 million. |
| During the fourth quarter of 2020 we reduced our estimate of the fair value of the adverse commitment by approximately $62.0 million based on our current manufacturing forecasts. Additionally, we recorded a reduction to our pre-tax loss of approximately $30.5 million due to a refund of interest paid associated with a tax matter. As of December 31, 2020, the cumulative loss on the divestiture of the Hillerød, Denmark manufacturing operations was $33.2 million. |
| | In late December 2018addition, we received feedback frommay earn certain contingent payments based on future manufacturing activities at the FDA regardingHillerød facility. For the designdisposition of a business, our policy is to recognize contingent consideration when the consideration is realizable. Consistent with our assessment as of the Phase 3 studiestransaction date, we currently believe the probability of vixotrigine for the treatmentearning these payments is remote and therefore we did not include these contingent payments in our calculation of TGN. Following this feedback, we are now planning to initiate the Phase 3 studies for our vixotrigine program for the treatment of TGN and, as a result, we adjusted the fair value of the operations. |
| | | | | | | | | A-2 | | 
| |  |
Appendix A (continued) | C | In February 2020 we entered into a collaboration and license agreement with Sangamo Therapeutics, Inc. (Sangamo) to develop and commercialize ST-501 for tauopathies, including Alzheimer’s disease; ST-502 for synucleinopathies, including Parkinson’s disease; a third neuromuscular disease target; and up to nine additional neurological disease targets to be identified and selected within a five-year period. In connection with the closing of this transaction in April 2020 we purchased $225.0 million of Sangamo common stock, or approximately 24 million shares at $9.21 per share, which are subject to transfer restrictions. We recorded an asset in investments and other assets in our contingent consideration obligations relatedcondensed consolidated balance sheets to reflect the initial fair value of the Sangamo common stock acquired and a charge of approximately $83.2 million to research and development expense in our vixotrigine programcondensed consolidated statements of income to reflect the premium paid for the treatmentSangamo common stock. We also made an upfront payment of TGN$125.0 million that was recorded as research and development expense. |
| D | In August 2020 we entered into a collaboration and license agreement with Denali Therapeutics Inc. (Denali) to co-develop and co-commercialize Denali’s small molecule inhibitors of leucine-rich kinase 2 (LRRK2) for Parkinson’s disease. As part of this collaboration, we purchased approximately $465.0 million of Denali common stock in September 2020, or approximately 13 million shares at $34.94 per share, which are subject to transfer restrictions. We recorded an asset in investments and other assets in our condensed consolidated balance sheets to reflect increased probabilitiesthe initial fair value of successthe Denali common stock acquired and recognized a losscharge of $80.6approximately $41.3 million to research and development expense in our condensed consolidated statements of income to reflect the fourth quarterpremium paid for the Denali common stock. We also made an upfront payment of 2018.$560.0 million that was recorded as research and development expense. |
| E | In June 2018November 2020 we closedentered into a newten-year exclusiveglobal collaboration and license agreement with Ionis Pharmaceuticals,Sage Therapeutics, Inc. (Ionis)(Sage) to jointly develop novel antisense oligonucleotide drug candidatesand commercialize BIIB125 (zuranolone) for a broad rangethe potential treatment of major depressive disorder, postpartum depression and other psychiatric disorders and BIIB124 (SAGE-324) for the potential treatment of essential tremor and other neurological diseases for a total paymentdisorders. In connection of $1.0 billion consistingthe closing of an upfront paymentthis transaction in December 2020 we purchased $650.0 million of $375.0 million and the purchase ofSage common stock, or approximately 11.56.2 million shares of Ionis’ common stock at a cost of $625.0 million. |
| The 11.5 million shares of Ionis’ common stock were purchased at a premium$104.14 per share, which are subject to their fair value at the transaction closing date. The premium consisted of acquiring the shares at a price above the fair value based on the trailing10-day weighted-average close price prior to entering into the agreement in April 2018 and the effect of certain holding periodtransfer restrictions. We recorded an asset of $462.9 million in investments and other assets in our consolidated balance sheets reflectingto reflect the initial fair value of the Sage common stock as of the purchase dateacquired and a charge of $162.1approximately $209.0 million to research and development expense in our consolidated statements of income during the second quarter of 2018 reflectingto reflect the premium paid for the Sage common stock. We also made an upfront payment of $875.0 million that was recorded as research and development expense.
|
| | | | | A-2 | |  | |  |
Appendix A(continued)
| F | In October 2017 we amended the terms of our collaboration and license agreement with Neurimmune SubOne AG (Neurimmune). Under the amended agreement, we made a $150.0 million payment to Neurimmune in exchange for a 15% reduction in the previously negotiated royalty rates payable on products developed under this agreement. In May 2018 we made an additional $50.0 million payment to Neurimmune to further reduce the previously negotiated royalty rates payable on products developed under this agreement by an additional 5%.
|
| Net distribution to noncontrolling interestIncome tax expense for the twelve months ended December 31, 2018, reflects2020, included $90.3 million in income tax expense related to a net valuation allowance against certain deferred tax assets, due to the $50.0 million payment made to Neurimmune, netdecisions of Neurimmune’s tax,the U.S. District Court of the Northern District of West Virginia and the U.S. District Court of the District of Delaware that the asserted claims of our U.S. patent No. 8,399,514, which cover the treatment of multiple sclerosis with 480 mg of dimethyl fumarate per day as provided for in May 2018.
|
| Net distribution to noncontrolling interest for the twelve months ended December 31, 2017, reflects the $150.0 million payment made to Neurimmune, net of Neurimmune’s tax, in October 2017.our TECFIDERA label, are invalid.
|
| G | 2017 corporate strategy implementation and restructuring charges are related to our efforts to create a leaner and simpler operating model.
|
H | Elimination of deferred tax asset due to Samsung Bioepis Co., Ltd. qualifying as a corporate joint venture for accounting purposes.
|
I | The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) resulted in significant changes to the U.S. corporate tax system. These changes include a federal statutory rate reduction from 35% to 21%, the elimination or reduction of certain domestic deductions and credits and limitations on the deductibility of interest expense and executive compensation. The 2017 Tax Act also transitions international taxation from a worldwide system to a modified territorial system and includes base erosion prevention measures onnon-U.S. earnings, which has the effect of subjecting certain earnings of our foreign subsidiaries to U.S. taxation as globallow-taxed income (GILTI). During the fourththird quarter of 20182019 a new taxing regime in the country and certain cantons of Switzerland was enacted, which we electedrefer to recognize deferred taxes for basis differences expected to reverse as GILTI is incurred and have established initial deferred tax balances, asSwiss Tax Reform. As a result of the enactment date of the 2017 Tax Act.
|
| During the fourth quarter of 2017 we recognized within our provision for income taxes a $1.2 billion provisional estimate pursuant to U.S. Securities and Exchange Commission Staff Accounting Bulletin No. 118. Our provisional estimate included an amount of $989.6 million associated with aone-time mandatory deemed repatriation tax on accumulated foreign subsidiaries’ previously untaxed foreign earnings (the Transition Toll Tax) and $184.0 million related to the impact of remeasuringSwiss Tax Reform, we recorded an income tax benefit of approximately $54.3 million resulting from a remeasurement of our deferred tax balances to reflect the new federal statutory rateassets and other changes to U.S. tax law.
|
| Tax reform amounts forliabilities in the twelve months ended December 31, 2018, reflect the effect of an expense of $135.8 million related to the establishment of GILTI deferred taxes.
|
| Tax reform amounts for the twelve months ended December 31, 2018, also reflect the effect of a net reduction of $34.6 million to our 2017 preliminary Transition Toll Tax estimate, an expense of $12.7 million for the remeasurement of our deferred tax balance and an $11.0 million expense to reflect other aspects of the 2017 Tax Act.
|
| The final determination of the Transition Toll Tax and remeasurement of our deferred assets and liabilities was completed in the fourth quarter of 2018.2019.
|
Use ofNon-GAAP Financial Measures We supplement our GAAP consolidated financial statements presented on aand GAAP basis by providing additional measures which may be considered“Non-GAAP” financial measures under applicable SEC rules.with other financial measures, such as adjusted net income, adjusted diluted earnings per share and free cash flow, which is defined as net cash flow from operations less capital expenditure. We believe that the disclosure of these and other Non-GAAP financial measures providesprovide additional insight into the ongoing economics of our business and reflectsreflect how we manage our business internally, set operational goals and form the basis of our management incentive programs. TheseNon-GAAP financial measures are in addition to, not a substitute for, or superior to, measures of financial performance prepared in accordance with generally accepted accounting principles in the United States and should not be viewed in isolation or as a substitute for reported, or GAAP, net income attributable to Biogen Inc., diluted earnings per share and net cash flows provided by operating activities.GAAP. Our“Non-GAAP net income attributable to Biogen Inc.” and“Non-GAAP earnings per share – Diluted” financial measures exclude the following items from “GAAP net income attributable to Biogen Inc.” and “GAAP earnings per share – Diluted”: 1. Purchase accounting, merger-relatedAcquisitions, divestitures and other adjustmentssignificant collaboration and licensing arrangements We exclude transaction, integration and certain purchase accountingother costs related to the acquisition and divestiture of businesses, the acquisitions of assets, significant collaboration and licensing arrangements and items associated with the acquisition of businesses, assets and amounts in relation to theinitial consolidation or deconsolidation of variable interest entities for which we are the primary beneficiary.entities. These adjustments include, but are not limited to, upfront payments in significant collaborations and licensing arrangements, charges for IPR&Din-process research and development and certain milestones, the amortization and impairment of intangible assets, and charges or credits from the fair value remeasurement of our contingent consideration obligations.obligations and losses on assets and liabilities held for sale. | | | | | | | A-3 | | A-3 | | 
| |  |
Appendix A(continued) 2. Hemophilia business separation costs We have excluded costs that are directly associated with the set up andspin-off of our hemophilia business on February 1, 2017. These costs represent incremental third-party costs attributable solely to hemophiliaspin-off and set up activities.
3. Restructuring, business transformation and other cost saving initiatives
We exclude costs associated with our execution of certain strategies and initiatives to streamline operations, achieve targeted cost reductions, rationalize manufacturing facilities or refocus R&Dresearch and development activities. These costs may include employee separation costs, retention bonuses, facility closing and exit costs, asset impairment charges or additional depreciation when the expected useful life of certain assets have been shortened due to changes in anticipated usage and other costs or credits that management believes do not have a direct correlation to our ongoing or future business operations. 4.3. (Gain) loss on equity security investments
Effective January 2018 weWe exclude unrealized and realized gains and losses and discounts or premiums on our equity security investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations.
5.4. Other items
We evaluate other items of income and expense on an individual basis and consider both the quantitative and qualitative aspects of the item, including (i) its size and nature, (ii) whether or not it relates to our ongoing business operations and (iii) whether or not we expect it to occur as part of our normal business on a regular basis. We also include an adjustment to reflect the related tax effect of all reconciling items within our reconciliation of our GAAP toNon-GAAP net income attributable to Biogen Inc. and diluted earnings per share. “Free Cash Flow” is defined as net cash flows provided by operating activities less purchases of property, plant and equipment and contingent consideration related to our acquisition of Fumapharm AG as disclosed in our 2018 Annual Report on Form10-K.share – diluted.
| | | | | | | A-4 | | A-4 | | 
| |  |
APPENDIX B Certificate of Amendment of Amended and Restated Certificate of Incorporation, as amended CERTIFICATE OF AMENDMENT TO THE CERTIFICATE OF INCORPORATION OF BIOGEN INC. Pursuant to Section 242 of the General Corporation Law of the State of Delaware Biogen Inc. (hereinafter referred to as the “Corporation”), a corporation duly organized and existing under the Delaware General Corporation Law (the “DGCL”), does hereby certify as follows: FIRST: That at a meeting of the Board of Directors of the Corporation on April 7, 2021, resolutions were duly adopted setting forth a proposed amendment to Article XII of the Certificate of Incorporation of said corporation, declaring said amendment to be advisable and directing that the proposed amendment be considered at the next annual meeting of the stockholders. As amended pursuant to such resolutions, Article XII of the Certificate of Incorporation shall be as follows: ARTICLE XII (a) Exclusive Forum. Unless the Board of Directors or one of its committees otherwise consents to the selection of an alternate forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action brought on behalf of the Corporation and (ii) any direct action brought by a stockholder against the Corporation or any of its directors or officers alleging a violation of the Delaware General Corporation Law, the Corporation’s certificate of incorporation or bylaws or breach of fiduciary duties or other violation of Delaware decisional law relating to the internal affairs of the Corporation; in each case excluding actions in which the Court of Chancery of the State of Delaware concludes that an indispensable party is not subject to the jurisdiction of the Delaware courts and can be subject to the jurisdiction of another court within the United States. (b) Federal Forum. Unless the Corporation consents in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. Any person or entity purchasing or otherwise acquiring any interest in any security of the Corporation shall be deemed to have notice of and consented to this provision. SECOND: The foregoing amendment was duly adopted in accordance with Section 242 of the DGCL. THIRD: The effective date of the amendment shall be June , 2021. IN WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment to be duly executed in its name this day of June, 2021. | | | | | | | | | B-1 | | 
| |  |
BIOGEN INC. 225 BINNEY STREET CAMBRIDGE, MA 02142 VOTE BY INTERNET Before The Meeting- Go towww.proxyvote.com Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. During The Meeting - Go towww.virtualshareholdermeeting.com/BIIB2019 BIIB2021 You may attend the Meeting via the Internet and vote during the Meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions. VOTE BY PHONE -1-800-690-6903 Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you call and then follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. | | | TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS:
| | E76108-P22124 TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: D48340-P53535 KEEP THIS PORTION FOR YOUR RECORDS | — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
|
DETACH AND RETURN THIS PORTION ONLY
FOR YOUR RECORDS THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. DETACH AND RETURN THIS PORTION ONLY BIOGEN INC. The Board recommends a vote FOR the following proposals: 1. Election of Directors. To elect the thirteen director nominees numbered 1a through 1m to serve for a one-year term extending until the 2022 annual meeting of stockholders and their successors are duly elected and qualified. For Against Abstain 1a. Alexander J. Denner 1b. Caroline D. Dorsa 1c. Maria C. Freire 1d. William A. Hawkins 1e. William D. Jones 1f. Nancy L. Leaming 1g. Jesus B. Mantas 1h. Richard C. Mulligan 1i. Stelios Papadopoulos 1j. Brian S. Posner 1k. Eric K. Rowinsky For Against Abstain 1l. Stephen A. Sherwin 1m. Michel Vounatsos 2. To ratify the selection of PricewaterhouseCoopers LLP as Biogen Inc.'s independent registered public accounting firm for the fiscal year ending December 31, 2021. 3. Say on Pay - To approve an advisory vote on executive compensation. 4. To approve an amendment to Biogen's Amended and Restated Certificate of Incorporation, as amended, to add a federal forum selection provision. For Against Abstain The Board recommends a vote AGAINST the following proposals: 5. Stockholder proposal requesting a report on Biogen's lobbying activities. 6. Stockholder proposal requesting a report on Biogen's gender pay gap. Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. Signature [PLEASE SIGN WITHIN BOX] Date Signature (Joint Owners) Date | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | BIOGEN INC. | | | | | | | | | | | | | | | | | | | | | | | | The Board recommends a voteFOR the following proposals: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 1. | | Election of Directors. To elect the fourteen director nominees numbered 1a through 1n to serve for a one-year term extending until the 2020 annual meeting of stockholders and their successors are duly elected and qualified. | | For | | Against | | Abstain | | | | | | | | | | For | | Against
| | Abstain
| | | | | | | | | | | | | | | | | | | | | | | 1a.
1b.
1c.
1d.
1e.
1f.
1g.
1h.
1i.
| | John R. Chiminski
Alexander J. Denner
Caroline D. Dorsa
William A. Hawkins
Nancy L. Leaming
Jesus B. Mantas
Richard C. Mulligan
Robert W. Pangia
Stelios Papadopoulos
| | ☐☐
☐
☐
☐
☐
☐
☐
☐
| | ☐☐
☐
☐
☐
☐
☐
☐
☐
| | ☐☐
☐
☐
☐
☐
☐
☐
☐
| | | | 1j.
1k.
1l.
1m.
1n.
| | Brian S. Posner
Eric K. Rowinsky
Lynn Schenk
Stephen A. Sherwin
Michel Vounatsos
| | | | ☐☐
☐
☐
☐
| | ☐☐
☐
☐
☐
| | ☐☐
☐
☐
☐
| | | | | | | | | 2.
| | To ratify the selection of PricewaterhouseCoopers LLP as Biogen Inc.’s independent registered public accounting firm for the fiscal year ending December 31, 2019.
| | ☐
| | ☐
| | ☐
| | | | | | | | | | | | | | | | 3.
| | Say on Pay - To approve an advisory vote on executive compensation.
| | ☐
| | ☐
| | ☐
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Signature [PLEASE SIGN WITHIN BOX] | | | | Date | | | | | | | | | | Signature (Joint Owners) | | Date | | | | | | |

Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting: The 20192021 Notice and Proxy Statement and 20182020 Annual Report with Form10-K are available at:www.proxyvote.com. — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
E76109-P22124
www.proxyvote.com. D48341-P53535 BIOGEN INC. Annual Meeting of Stockholders June 19, 2019,2, 2021, 9:00 a.m. Eastern Time This proxy is solicited by the Board of Directors The undersigned hereby appoints Michel Vounatsos, Jeffrey D. CapelloMichael R. McDonnell and Susan H. Alexander, and each of them (with full power to act alone), as proxies of the undersigned with all the powers the undersigned would possess if present during the 20192021 Annual Meeting,and with full power of substitution in each of them to appear, represent and vote all shares of common stock of Biogen Inc. which the undersigned would be entitled to vote at the 20192021 Annual Meeting of Stockholders, to be held at Biogen Inc.’s offices located at 225 Binney Street, Cambridge, Massachusetts 02142 and online at www.virtualshareholdermeeting.com/BIIB2019BIIB2021 on Wednesday, June 19, 2019,2, 2021, at 9:00 a.m. Eastern Time, and at any adjournment or postponement thereof. The shares represented by this proxy will be voted as directed herein. If no direction is indicated, such shares will be voted FOR the election of all of the director nominees listed in Proposal 1, and FOR Proposals 2, 3 and 3.4 and AGAINST Proposals 5 and 6. As to any other matter that may be properly brought before the meeting or any adjournment or postponement thereof, proxy holders will vote in accordance with their best judgment. Continued and to be signed on reverse side |
|
|
|
| |
|
|
|
|
|



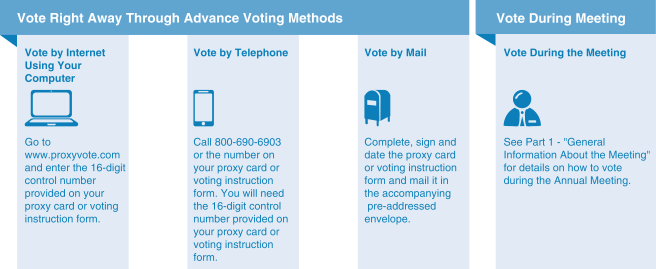
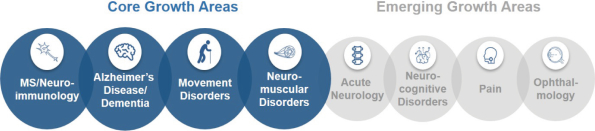
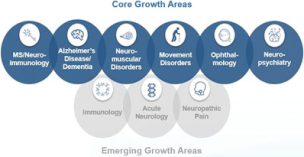










 By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted throughwww.proxyvote.com must be received by 11:59 p.m. Eastern Time on June
By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted throughwww.proxyvote.com must be received by 11:59 p.m. Eastern Time on June 
 By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted by telephone must be received by 11:59 p.m. Eastern Time on June
By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted by telephone must be received by 11:59 p.m. Eastern Time on June 
 By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June
By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June 
 During the Annual Meeting. You may vote during the Annual Meeting by
During the Annual Meeting. You may vote during the Annual Meeting by 






























































 Target Setting
Target Setting
 Monitoring & Tracking
Monitoring & Tracking Results & Awards:
Results & Awards:

 Target Setting
Target Setting
 Monitoring & Tracking
Monitoring & Tracking Results & Awards:
Results & Awards:







